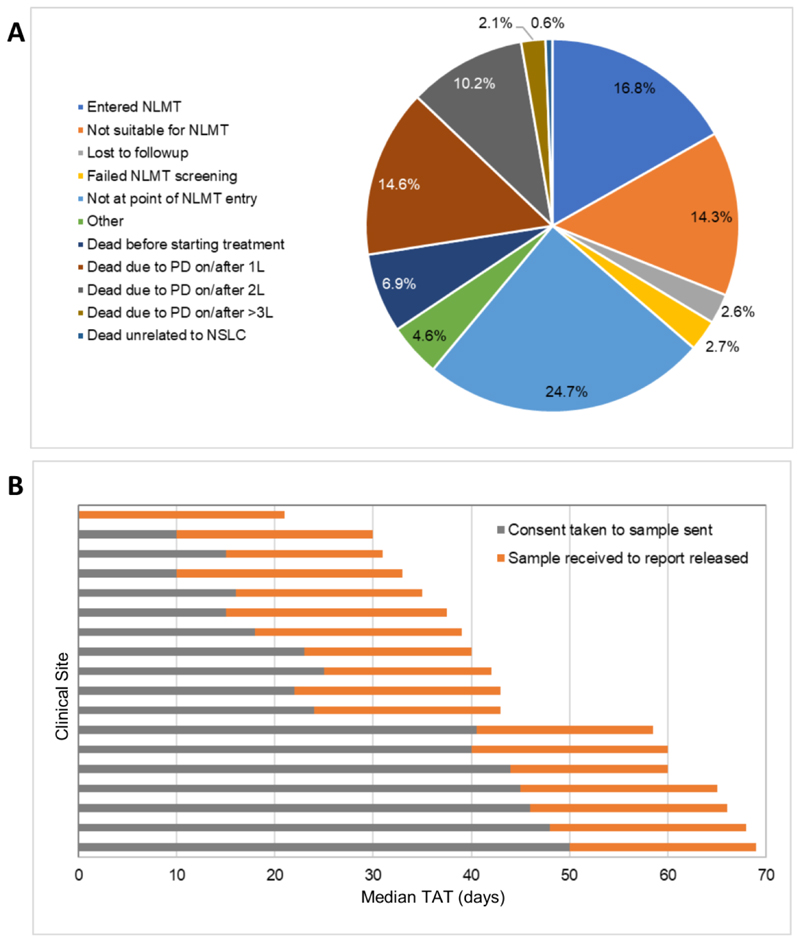
Extended Data Fig. 1. Reasons for attrition and median testing turnaround time in the SMP2 study.
a, Reasons why patients enrolled in SMP2 did not enter the NLMT were collected for a subset of patients (N=1433). PD, progressive disease; 1L, first-line treatment; 2L, second-line treatment; 3L, third-line treatment. b, Median turnaround time (TAT) of SMP2 testing. Turnaround time was measured in days from the 18 SMP2 clinical sites that recruited patients. This consists of the median time from when informed consent was received from the patient to enter SMP2 to the tissue sample being sent for testing (grey bars) and from receipt of the tissue sample at the SMP2 technical hubs to the release of the SMP2 screening report (orange bars).