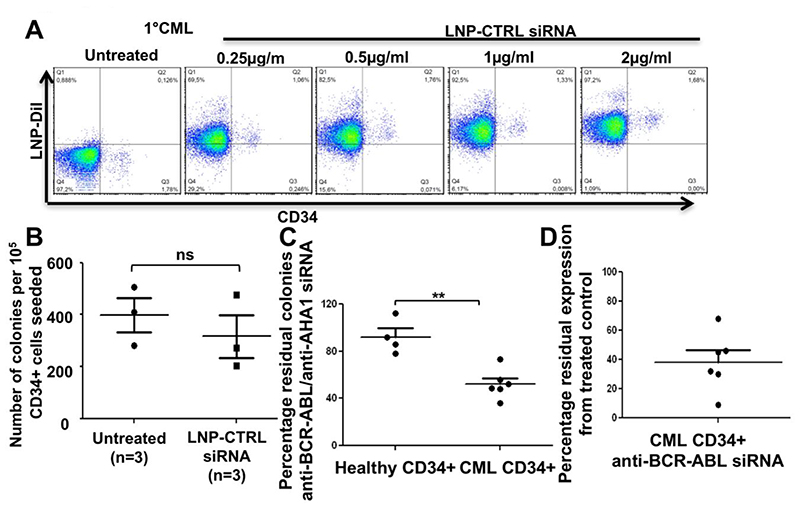
Figure 4. Efficient uptake and on target efficacy of LNP-siRNA in primary human CD34+ CML cells.
A. Representative FACS plot for uptake of LNP-CTRL siRNA in CD34 negative and CD34 positive CML bone marrow cells at day 3 after a single treatment of different concentrations of LNP-CTRL siRNA in vitro (representative of n=3 CML samples).
B. Colony number from CFC assays with healthy human CD34+ cells either untreated or treated with LNP-CTRL-siRNA (1μg/ml). 100,000 cells were plated in 3 mL semi-solid medium and 1μg/ml LNP-CTRL-siRNA was added to this medium. Colony numbers were counted after 14 days of incubation (mean ± SEM, n=3).
C. Residual colony number from CFC assays with healthy human CD34+ cells or CD34+ CML patient cells cells treated with LNP-siRNA (CTRL or BCR-ABL). 100,000 cells were treated overnight with LNP-CTRL-siRNA or LNP-BCR-ABL-siRNA (1μg/ml each) and plated in 3 mL semi-solid medium. Colony numbers were counted after 14 days of incubation (mean ± SEM, n=4 CD34+ healthy controls and n=6 CD34+ primary CML patient cells).
D. RT-PCR validation of BCR-ABL knockdown in CD34+ primary CML cells treated with LNP-siRNA (CTRL or BCR-ABL). 100,000 cells were treated with LNP-CTRL-siRNA or LNP-BCR-ABL-siRNA (1μg/ml each) for 72 hrs (mean ± SEM, n=6 CD34+ primary CML patient cells).