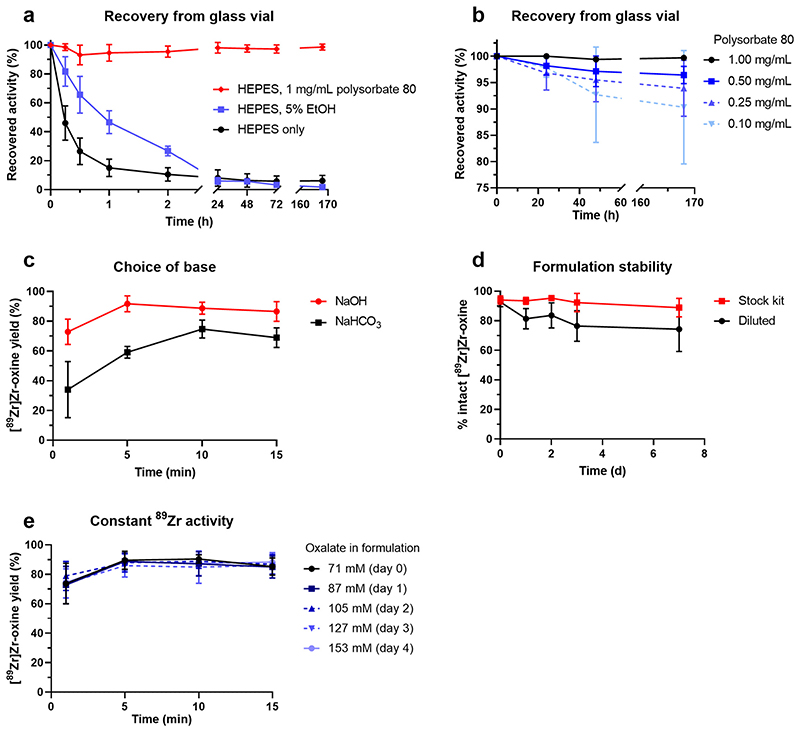
Fig. 2. Formulation optimisation for [89Zr]Zr-oxine.
(a, b) Recovery: percentage of [89Zr]Zr-oxine remaining in solution over time when formulated in HEPES buffer only and in presence of 5% EtOHorvarying concentrations of polysorbate 80. Mean ± SDof n = 3 separate experiments. (c) Yield of [89Zr]Zr-oxine over time in HEPES buffers (pH 7.9) containing NaOH (n = 5) or NaHCO3 (n = 4), measured by radioTLC. (d) Stability: [89Zr]Zr-oxine as percentage oftotal89Zr activity in solution over 7 days, measured by radioTLC (n = 3), in original 100 pLformulation or after 10-fold dilution with H2O. (e) Formation rate and radiochemical yield of [89Zr]Zr-oxine as a function of oxalate content and 89Zr decay. [89Zr]Zr-oxalate in 1 M oxalic acid (1.1-1.5 MBq/μL on day 0, approximately 4-5 days after production in the cyclotron) was added to 20 μL aliquots of kit formulation on day of reception (day 0) and 4 subsequent days. To keep total 89Zr activity constant, increasing volumes of [89Zr]Zr-oxalate solution were added on each day, resulting in increasing concentrations of oxalate ions in the final product. Mean ± SD of n = 3 separate experiments.