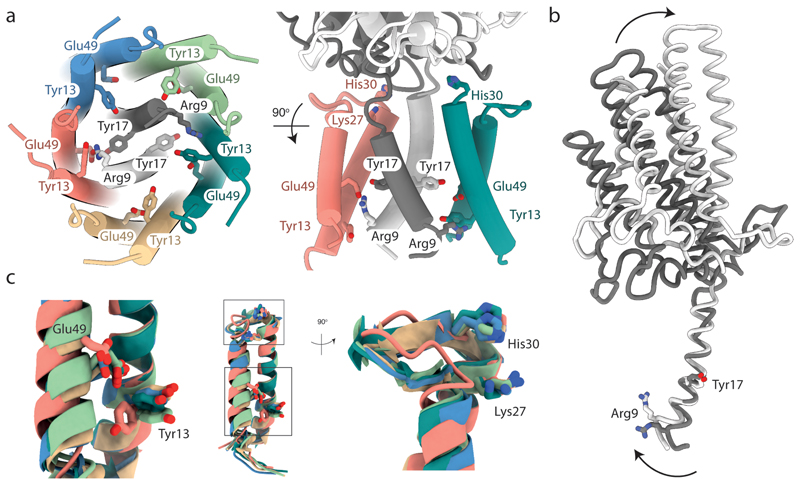
Fig. 5. The structural asymmetry of the GldLM′ complex leads to important differences in residue environments between chains.
The side chains of functionally important resides within the transmembrane domain of GldLM are shown in stick representation with oxygen atoms red and nitrogen atoms blue. Note that the side chains of Glu49 are absent from the EM density due to decarboxylation. The side chain of Glu49 in the salmon chain (chain C) is modelled on the basis that it is inferred to form an ion pair with Arg9 in the white GldM chain (chain A). The modelled side chain positions of Glu49 in the other GldL chains are arbitrary. Proteins chains are coloured as in Fig. 2 a-c.
a, The side chains of functionally important resides. For clarity, only two GldL subunits are shown in the right hand view.
b, Overlay of the two copies of GldM′ by superimposition of the TMH. Arg9 in the white chain (chain A) is involved in the ion pair with GldL chain C Glu49 shown in a. Arrows indicate the conformational changes between the two GldLM chains.
c, Superimposition of the five copies of GldL by overlaying TMH2. The boxes in the central panel indicating the positions of the two regions magnified on either side.