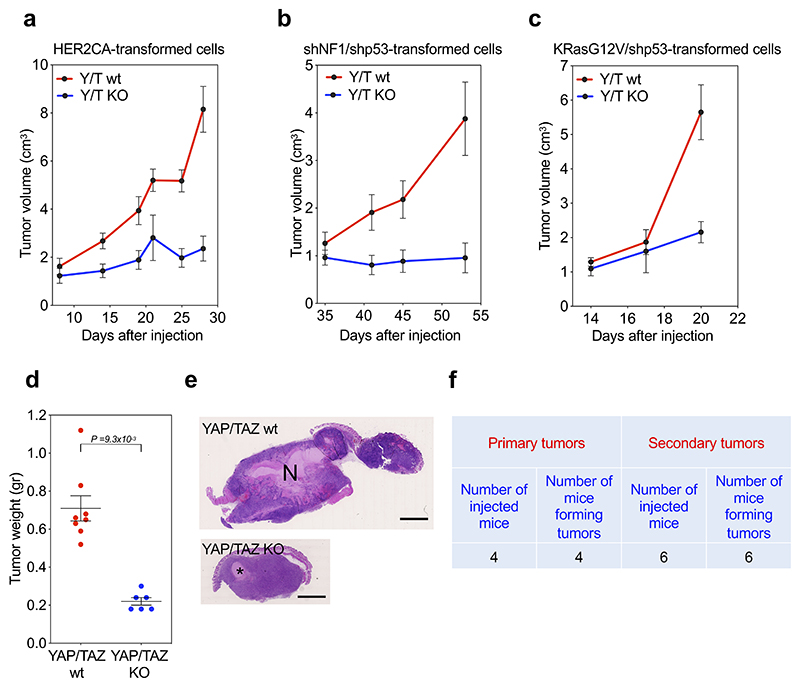
Extended Data Fig. 6. YAP/TAZ are required for GSC maintenance in vivo.
(a-c) Effects of YAP/TAZ knockout on the growth of established subcuteaneous GBM-like lesions. Transformed cells were obtained by dissociation of gliomaspheres obtained from HER2CA- (a), shNF1/shp53- (b) or KRasG12V/shp53- (c) transformed R26CAGCreERT2; Yapfl/fl; Tazfl/fl newborn mouse astroglial cells (as in Fig. 3), and then injected in NOD-SCID mice. When subcutaneous tumors reached approximately 0.5 cm of diameter, mice were either fed with Tamoxifen food to induce YAP/TAZ knockout (YAP/TAZ KO), or maintained under normal diet (YAP/TAZ wt). Graphs are growth curves of YAP/TAZ wt (KRasG12V/shp53-, n=4 mice; HER2CA, n=6 mice; shNF1/shp53, n=5 mice) and YAP/TAZ KO (KRasG12V/shp53-, n=4 mice; HER2CA, n=4; shNF1/shp53, n=8 mice) tumors (average volume ± s.e.m.).
(d, e) Effects of YAP/TAZ knockout in tumors derived from KRasG12V/shp53 gliomaspheres, following the experimental setup described in a-c. (d) Dot plot for tumor weight at sacrifice (YAP/TAZ wt, n=8; YAP/TAZ KO, n=6). Mean ± s.e.m. of the distribution are also shown. p-value was calculated by unpaired two-tail t-test. (e) Representative H&E stainings. Scale bar, 2.5 mm. N, necrotic area; *, Matrigel residue.
(f) Tabular results showing the number of NOD/SCID mice displaying subcutaneous tumor formation after injection of cells dissociated either from gliomaspheres derived from HER2CA-transformed primary newborn astroglial cells (Primary tumors), or from HER2CA-gliomaspheres derived from one of the Primary tumors (Secondary tumors).