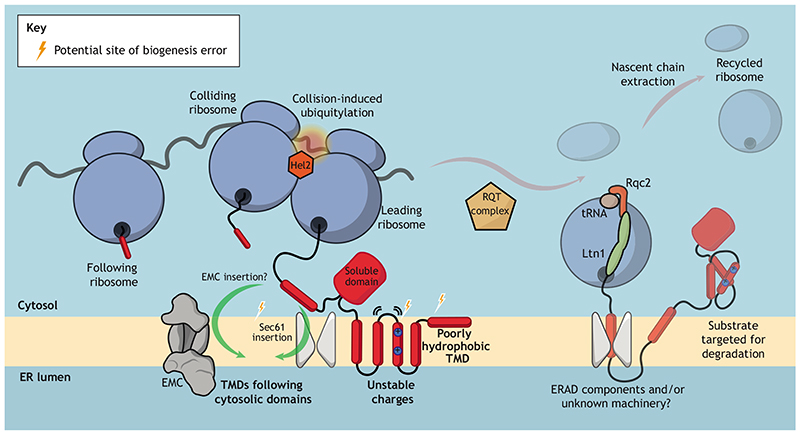
Fig. 2. Membrane protein biogenesis defects result in ER-RQC.
Defects in TMD insertion through the EMC or Sec61, attempted insertion of poorly hydrophobic TMDs or poor shielding of TMD charges might all contribute to ribosome stalls. Additionally, insertion of TMDs after large soluble domains might also present a challenge due to release of the ribosome from the translocon while cytoplasmic domains are synthesised. Translational stalls at the ER that result in ribosome collisions are recognised by the ubiquitin ligase Hel2, followed by splitting of the ribosome by the RQT complex (and potentially also Dom34-Hbs1). Following splitting of the ribosome, the exposed nascent chain-tRNA complex is recognised by Rqc2 and listerin (Ltn1). This complex ubiquitylates the nascent chain, targeting it for degradation. The final step of extraction and degradation of the chain is less well understood, and evidence concerning details of this process at the ER is sparse. Extraction and degradation of substrates at the ER may involve as yet unidentified proteins, potentially including ERAD machinery.