Abstract
This study investigated contaminant transport and evaluated the ventilation performance in a single-bed inpatient room. The study performed comparative experimental analysis on the distributions of respiratory contaminants breathed out and coughed out by a patient in a full-scale chamber, which simulated a single-bed inpatient room. The contaminant exhaled by the patient was simulated by an SF6 tracer gas and 3-μm particles at steady-state conditions. The differences in the contaminant distribution between the coughing and breathing cases were insignificant for the mixing ventilation case, while for the displacement ventilation, the contaminant concentrations in the upper part of the room were higher for the coughing case. The contaminant concentrations in the inpatient room for the case with the patient sitting on the bed were lower than those for the patient supine on the bed for the displacement ventilation under the same supply airflow rate. The SF6 tracer gas and 3-μm particles released at a notable initial velocity for simulating a cough could give similar contaminant distributions in the inpatient room. Therefore, the experimental data can be used to validate a CFD model, and the validated CFD model can be used to investigate transient coughing and breathing processes.
Keywords: Displacement ventilation, Mixing ventilation, Inpatient room, Cough, Breath, Posture
1. Introduction
Respiratory infectious diseases, such as Severe Acute Respiratory Syndrome (SARS), could be transferred from a patient to other people in the proximity by air [1]. Many infections may have been the result of poor ventilation and insufficient protection of healthcare workers [2]. The experience with SARS indicated a disease transmission in a short route between healthcare workers and patients. Therefore, it is important to understand air and contaminant transport in inpatient rooms and to improve the ventilation system design for the rooms.
Many studies have been conducted on airborne disease transmission and ventilation systems in hospitals. Li et al. [3] reviewed the literature in the last half century and concluded that there is an association between the infection caused by airborne disease transmission and the ventilation in buildings. However, there is insufficient data to specify and quantify the minimum ventilation requirements in hospitals. Tang et al. [4] pointed out many important factors involved in the aerosol transmission of infectious diseases, including aerosol generation from breathing, coughing, and talking. They indicated that it should be possible to reduce the risk of aerosol transmission by altering the ventilation parameters in healthcare environments. This has been verified by our previous study [5] which showed that ventilation systems play a very important role in the contaminant distribution in an inpatient room. Qian et al. [6] indicated that the downward laminar airflow pattern recommended by the CDC was impossible to achieve due to turbulent flow mixing and thermal buoyancy. To prevent or minimize the spread of airborne infection, the physical characteristics of the indoor environment and the design and operation of building ventilation systems are critical [7]. Patients in hospital wards often generate airborne contaminants by different expiration modes, such as coughing, breathing, talking, and sneezing. Many experimental studies on coughing have showed that the average velocity of coughing varied from 1 to 10 m/s [8], while the average velocity of breathing is low so it can be treated as a stationary source. Thus, the contaminant is released at very different velocities in coughing and breathing. Transport of the contaminant exhaled by coughing should be different from that by breathing. Zhu et al. [9] used a CFD method to investigate the flow field and infection risk influenced by realistic coughing and breathing of occupants in a stagnant indoor environment. Their results showed that indoor ventilation conditions can greatly affect the chances of airborne infections by viruses. Melikov and Kaczmarczyk [10] performed a measurement of indoor air quality using a breathing thermal manikin in a room with an underfloor air distribution system. The results showed that the body surface temperature and posture were very important in exposure to the contaminants.
Patients often occupy a bed in an inpatient room using different postures, such as supine or sitting. Sun and Ji [11] investigated numerically the transport and dispersion of particles of different sizes exhaled by a lying/sitting person on a bed in a room that had mixing and displacement ventilation. The simulation results showed that the coughing direction had an obvious effect on the dispersion of different-size particles in an unsteady condition. Particularly for the particles with a size of less than 100 μm, the transport and dispersion showed complex behavior in the air-conditioned room. Previous work on coughing and breathing focused on the transient dispersion and analyzed the infectious risk just between persons [12], [13]. But little has been done on contaminant removal by ventilation systems.
This investigation used an environmental chamber to simulate a one-person inpatient room, in which the patient could breathe or cough out infectious disease viruses at lying and sitting postures. This paper reported our study on (1) the difference in contaminant distributions generated by the patient in breathing and coughing expiration modes with displacement ventilation; (2) the exposure risk of a healthcare worker in the room with different ventilation systems; and (3) the effect of patient postures on contaminant distributions.
2. Experimental setup and method
This investigation used an environmental chamber to simulate a full-scale, one-person inpatient room (4.90 m × 4.32 m × 2.72 m) as shown in Fig. 1 . The room was furnished with one bed, cabinets, a TV set with 23.8 W of heat load, a piece of medical equipment with 36.3 W of heat, a simulated patient with 106.3 W of heat supine on the bed, and a simulated healthcare worker with 110.6 W of heat. The room used a four-way overhead air-supply diffuser that generated mixing ventilation or employed a perforated diffuser on the floor at the end wall that produced displacement ventilation. There were two ventilation exhausts in the room. One was the main exhaust and the other was an auxiliary exhaust through the restroom. The ventilation rates studied were 4 ACH (0.057 m3/s) and 6 ACH (0.085 m3/s), respectively. The auxiliary exhaust extracted 0.039 m3/s of air from the inpatient room to the restroom, while the main exhaust extracted the rest. The 6 ACH ventilation rate was used in the mixing ventilation, which is the standard supply flow rate for inpatient rooms. The lower ventilation rate was used for displacement ventilation since it could provide better air quality than mixing ventilation. The supply air temperature was 19.5 °C for both systems. This investigation studied the distributions of the contaminant exhaled by a patient with sitting and supine postures on a bed and with different expiration modes (breathing and coughing) in the room.
Fig. 1.
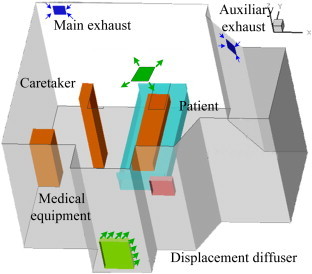
The full-scale inpatient room with displacement and mixing ventilation systems.
This study conducted seven cases of measurements as summarized in Table 1 . These cases were designed to investigate the distributions of contaminants exhaled by a patient with different postures (sitting and supine), different expiration modes (breathing and coughing), and different ventilation systems (mixing and displacement ventilation). Cases 1 and 4 compared breathing with coughing for the displacement ventilation and Cases 2 and 3 for the mixing ventilation. Cases 5 and 1 and Cases 6 and 7 studied the impact of the ventilation rate on the contaminant distribution in the room with displacement ventilation.
Table 1.
Cases studied in this investigation.
| Cases | Ventilation | Flow rate (ACH) | Posture | Respiratory mode | Contaminants |
|
|---|---|---|---|---|---|---|
| Tracer gas | Particle | |||||
| 1 | Displacement | 4 | Lying Supine | Breathing | Yes | No |
| 2 | Mixing | 6 | Lying Supine | Breathing | Yes | Yes |
| 3 | Mixing | 6 | Lying Supine | Coughing | Yes | Yes |
| 4 | Displacement | 4 | Lying Supine | Coughing | Yes | Yes |
| 5 | Displacement | 6 | Lying Supine | Breathing | Yes | No |
| 6 | Displacement | 4 | Sitting | Breathing | Yes | No |
| 7 | Displacement | 6 | Sitting | Breathing | Yes | No |
Note: SF6 and 3-μm particles used as the gaseous and particulate contaminants.
Both gaseous and particulate contaminants were exhaled by the patient in the room. A patient with an airborne infectious disease often releases respiratory viruses into the surroundings through breathing and coughing. Both breathing and coughing are transient processes. However, it is difficult to make a transient measurement in an inpatient room because a cough exhalation lasts for less than 1 s [8] and a breathing cycle takes around 5 s, but measurements of contaminant concentration need around 10–20 s. Hence, our investigation simplified the coughing and breathing as constant processes, which means that the virus release was simulated at a constantly releasing rate. Coughing differs from breathing in velocity and direction. Coughing has a distinguished momentum and direction. Therefore, for simplicity, our study simulated breathing by introducing a contaminant source at negligible velocity.
The usual sizes of the droplet nuclei exhaled by people differ much depending on the expiration modes [14], however, droplets with big size would deposit to walls or floor very soon. The main size of the droplet transporting in air is in the range of 0.8–3 μm [15]. To simulate infectious disease viruses breathed out by a patient, this study used a tracer gas, sulfur hexafluoride or SF6, and a monosize particle of 3 μm. Both of the contaminants were released at a constant rate at the mouth position of the patient. The studies by Chao et al. [16] indicated that the deposition fraction for this size range was less than 20% for 6 ACH flow in a hospital environment. Therefore, the current study assumed no deposition. The SF6 tracer gas was in a 1% solution and was introduced at a constant flow rate of 300 mL/min. This investigation did not use large particles as they may be deposited quickly on the floor and may not be airborne. The releasing rate of the particles depended on the background concentration of the particles in the supply air and room air. Our experiment adjusted the releasing rate to ensure that the particle concentration in the room would be at least 100 times higher than the background concentration. This would eliminate the influence of the background particle concentration on the data. The particles were generated by a condensation Monodisperse Aerosol Generator (TSI Model 3475).
To simulate the disease viruses from breathing, the SF6 was introduced into a sponge sphere with a diameter of 4 cm, so the tracer gas was released into the room at a very low velocity and at a constant releasing rate. To simulate the disease viruses from coughing, a “coughing machine” was used that had a fan mixing the room air with SF6 gas or particles, as shown in Fig. 2 (a). The outlet velocity of the coughing machine was 2.9 m/s, which was obtained by integrating one of the cough flow rates from the studies by Gupta et al. [8] and dividing it with the mouth opening area and the “mouth opening” was round, with a diameter of 0.02 m. The coughing position had a 50° angle upwards for the patient lying on a bed, as shown in Fig. 2(b).
Fig. 2.
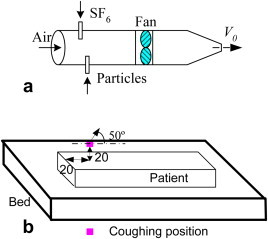
(a) A “coughing machine” and (b) the coughing position and direction.
To investigate the effect of different postures of a patient on contaminant distributions and the infection risk to the healthcare worker, two typical postures (supine and sitting) were studied, as shown in Fig. 2(b) and Fig. 3 , respectively.
Fig. 3.
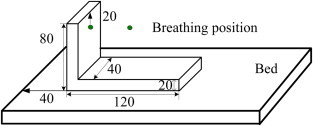
A patient sitting on the bed (unit: cm).
All the measurements were conducted under steady-state conditions. This implies that the breathing and coughing processes were continuous, which is of course not true in reality. However, the steady state would help to establish with high accuracy stable conditions for measurements.
The experiment measured the air velocity and temperature with omni-directional anemometers at 3 poles (VT1, VT2, and VT3, as shown in Fig. 4 ). The measurements were conducted at seven heights in each pole, namely 0.12, 0.35, 0.85, 1.35, 1.85, 2.35, and 2.60 m above the floor. The SF6 and particle concentrations were measured in five poles in the room (TG1 through TG5, as shown in Fig. 4) at six different heights above the floor (0.12, 0.60, 1.10, 1.60, 2.10, and 2.60 m above the floor). A photoacoustic multi-gas analyzer (INNOVA model 1312) with a multipoint sampler (INNOVA model 1309) was used to measure the SF6 concentration with an accuracy of 0.001 ppm. An aerodynamic particle sizer spectrometer (TSI model 3321) was used to measure the particle concentration with a size range from 2.642 to 3.523 μm for the 3-μm particles.
Fig. 4.
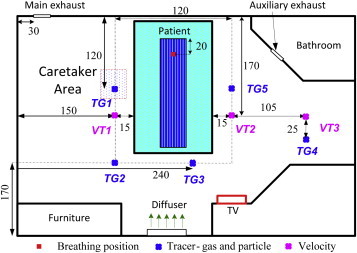
Measurement locations: VT1–VT3 for velocity and temperature and TG1–TG5 for tracer gas and particles (unit: cm).
3. Results
3.1. Air distributions
Fig. 5 shows the air velocity at VT1, VT2, and VT3 locations with different ventilation systems when the patient was supine on the bed. The results showed that the air velocity in the room with mixing ventilation was significantly higher than the velocity in the displacement ventilation in most areas. The air velocities in the room with displacement ventilation under different ventilation rates were almost the same except for the area close to the floor, marked in Fig. 5, although the supply airflow rates were different. This indicates that the flow in the room with displacement ventilation was mainly created by thermal buoyancy, not the momentum from the diffuser. In fact, the air velocity from the diffuser was very low. Fig. 6 shows the airflow velocity profiles at several locations 5 cm away from the diffuser surface at an airflow rate of 4 ACH. The diffuser was supposed to produce a uniform airflow on its surface. However, the results showed that the velocity in the lower part of the diffuser was much higher than the velocity in the upper part. This is because the thermal buoyancy between the cool supply air and the warm room air caused a downward flow.
Fig. 5.
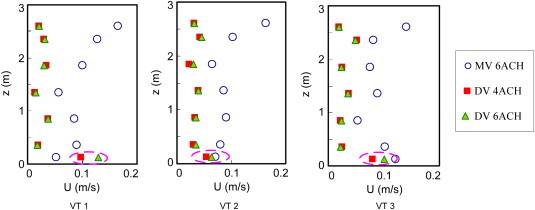
Air velocity profiles at VT1, VT2, and VT3 positions in the room (DV – displacement ventilation; MV – mixing ventilation; z – height (m); and U – velocity (m/s)).
Fig. 6.
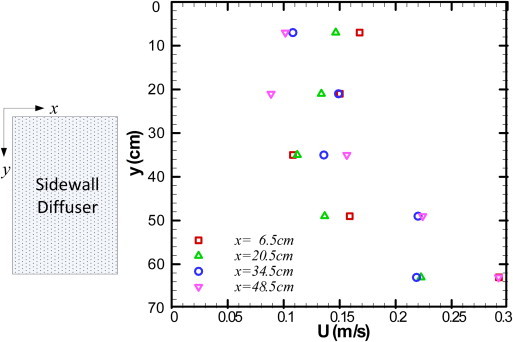
Air velocity distributions at 5 cm from the diffuser surface for the displacement ventilation.
3.2. Contaminant distributions in the inpatient room with different expiration modes from the patient
Typical displacement ventilation uses an air supply rate of 4ACH and would produce a stratified flow in the room, which is considered to provide better ventilation performance than mixing ventilation. However, in an inpatient room, a patient would generate contaminants by breathing or coughing. The objective of this task was to identify the differences caused by the different expiration modes. This study compared a breathing case (Case 1) and a coughing case (Case 4) under the displacement ventilation at a 4ACH airflow rate and also used another breathing case (Case 2) under the mixing ventilation for comparison.
Fig. 7 shows the concentration distributions of the contaminant generated by different expiration modes in the room. The results indicated that the displacement ventilation produced stratified airflow in both the breathing and coughing cases. In both cases, the contaminant (tracer gas) concentration profiles in the area with a height below 1 m in the room were similar, but differed greatly in the upper area of the room. In the coughing case, the displacement ventilation system would produce high contaminant concentration in the upper part of the room compared with that in the breathing case. The high concentrations were caused by the upward flow from the coughing machine. However, in the mixing ventilation, the differences in the contaminant distributions were small when the patient coughed or breathed, as shown in Fig. 8 .
Fig. 7.
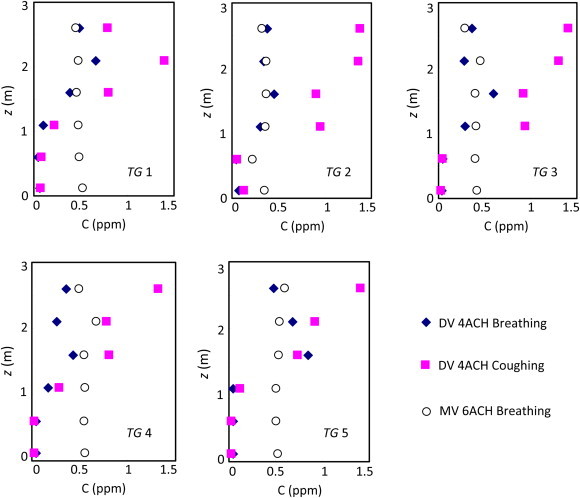
Contaminant concentrations in the inpatient room generated by different expiration modes (DV – displacement ventilation; MV – mixing ventilation; Case 1 – DV 4ACH breathing; Case 2 – MV 6ACH breathing; and Case 4 – DV 4ACH coughing).
Fig. 8.
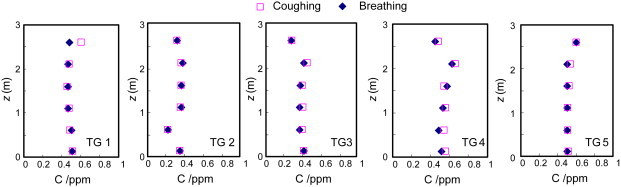
Contaminant concentrations in the inpatient room generated by coughing and breathing in mixing ventilation.
Case 1 was for the displacement ventilation with an airflow rate of 4 ACH and Case 2 for the mixing ventilation with an airflow rate of 6 ACH. The contaminant concentration profiles differed little in the inpatient room with the mixing ventilation due to the strong mixing of the air. At the breathing zone of a standing person (z =1.5 m), the contaminant concentration (tracer gas) was almost the same between the two ventilation systems for the breathing case. In some areas such as TG3 and TG5 the displacement ventilation may lead to a slightly higher contaminant concentration than in the mixing ventilation.
3.3. Contaminant distributions in the inpatient room with different patient postures
This study also investigated the contaminant distribution in the inpatient room with the patient breathing out the contaminant in different postures. In Case 7, the patient was in a sitting position and in Case 5 in supine position. Both cases had the same supply airflow rate of 6 ACH. A lower supply airflow of 4ACH was used for Case 6. Fig. 9 displays the contaminant concentration simulated by tracer gas for the three cases. The results indicated that in a space with a height of less than 2 m, the contaminant concentration in the sitting case was lower than that in the supine case. This was due to the contaminant source location in the sitting case being higher than that in the supine case, and it was easier for the contaminant removal through the high level exhaust when the source location was higher. Comparing the two sitting cases (Case 6 and Case 7), the case with a lower supply airflow rate showed higher contaminant concentration in the inpatient room. This is obvious but the improvement in the air quality was not proportional to the increase in the ventilation rate. The case with the patient in a supine position showed similar results.
Fig. 9.
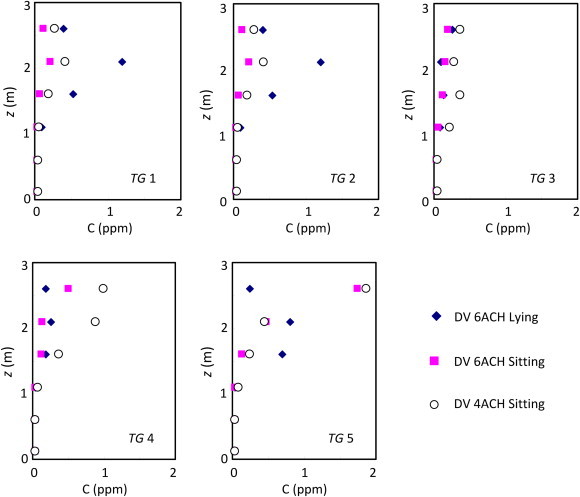
Concentration of contaminants generated by the patient with different postures in the room with displacement ventilation (DV – displacement ventilation; MV – mixing ventilation; Case 5 – DV 6ACH lying; Case 6 – DV 4ACH sitting; and Case 7 – DV 6ACH sitting).
3.4. Simulation of contaminant by tracer gas and particles due to the coughing cases in the inpatient room
Our previous study [5] concluded that the tracer gas and 3-μm particles can be used to simulate contaminant release from a patient if the release is at a negligible velocity. This type of release includes breathing and talking. However, if the release is by coughing and sneezing, the source has significant momentum.
Cases 3 and 4 were setup for mixing and displacement ventilation. In each case, both tracer gas and 3-μm particles were released simultaneously from the coughing machine (shown in Fig. 2(a)). Considering the differences in the releasing rates, this investigation used a dimensionless concentration for comparison:
| (1) |
where C p and C s are the tracer-gas or particle concentration at the measured location and the air supply inlet, respectively. In this study, the supply air is fresh air with zero tracer gas concentration and C e is the averaged tracer-gas or particle concentration at the two exhausts determined by
| (2) |
where G s is the supply airflow rate and RC is the releasing rate of the tracer gas or particles.
Fig. 10 compares the concentration profiles of the SF6 and 3-μm particles for the coughing cases. Although the contaminant source was introduced with a notable initial velocity, the SF6 profiles were similar to those of the 3-μm particles. This confirms that both the tracer gas and 3-μm particles released at a large initial momentum can be used to simulate a contaminant. Our experience shows that the tracer gas is much easier to use than the particles, and the accuracy also seems higher.
Fig. 10.
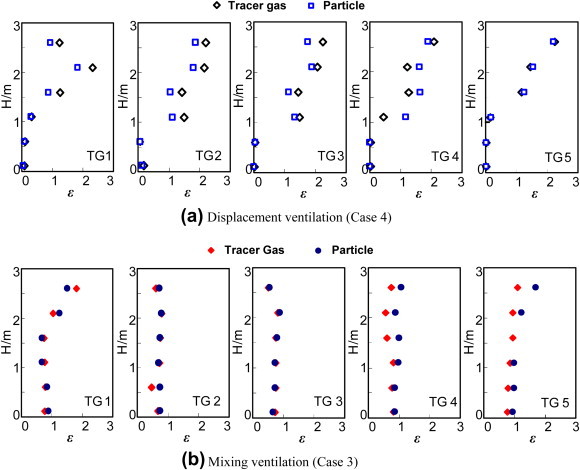
Comparison of the contaminant profiles for the coughing cases using SF6 gas and 3-μm particles. (a) Displacement ventilation; and (b) Mixing ventilation.
4. Discussion
The exhaled air from coughing has a higher velocity and can contain more contaminants than those from breathing. But the coughing process is an impulse event, while breathing is periodic and lasts longer. Thus, it is important to investigate both processes. The current study considered these processes as continuous and steady, which was a major simplification of the processes. Coughing and breathing processes are transient and if modeled as unsteady events can provide information on transient movement of contaminants. The released contaminants will eventually leave the room after a while. The current investigations modeled these exhalations as steady sources and thus provided information on final distribution of contaminants in the room for a steady release. Therefore, the concentration values obtained from steady case will be different from the transient case but the movement of the contaminant will be similar as the source airflow will not significantly affect the airflow in most of the room. Thus the steady case can still provide qualitative information on contaminant distribution. The breathing and coughing exhalation processes are transient, and a transient CFD simulation should be performed to predict the transport of contaminants released from these exhalations. However, CFD simulations need validation by experimental data. In the absence of experimental data on transient transport of contaminants, a steady-state CFD simulation can be performed and validated by using our experimental data. Then the CFD could be used to study transient contaminant transfer in an inpatient room. The current investigation assumed that the momentum of contaminants released during breathing was negligible. This may under-predict the breathing jet penetration. The peak velocity of a cough could be higher than the averaged velocity of cough used in the current study. Thus the momentum of contaminants at the peak velocity would be higher than that used in the current investigation. Therefore, this may have under-predicted the jet penetration due to cough. A transient study with real coughing and breathing by experiment is difficult, so it is a subject for future studies.
5. Conclusions
This investigation conducted an experimental study on contaminant transmission from a patient in an inpatient room by using a full-scale mockup. The main findings in this study are:
-
(1)
In the mixing ventilation, the differences in the contaminant distributions were small when the patient coughed or breathed, while in the displacement ventilation, the coughing case would cause a significantly higher concentration of the contaminant in the upper part of the room than would the breathing case.
-
(2)
The contaminant concentration in the room with the displacement ventilation was higher for the case with the patient in a supine posture than in a sitting posture.
-
(3)
Even if the contaminant had been released with a notable initial velocity (such as coughing), the SF6 tracer gas simulated a very similar contaminant distribution in the inpatient room as the 3-μm particles. Since it is much easier to use tracer gas for an experiment than particles, tracer gas is recommended unless a contaminant contains mainly large particles.
Acknowledgments
The first author would like to thank Purdue University for hosting him as a Visiting Scholar at the time this study was conducted. The work was partially supported by China’s Scholarship Council and the Natural Science of Foundation of China (No. 50976021).
References
- 1.Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.W. Evidence of airborne transmission of the severe acute respiratory syndrome virus. New England Journal of Medicine. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 2.Richmond-Bryant J. Transport of exhaled particulate matter in airborne infection isolation rooms. Building and Environment. 2009;44(1):44–55. doi: 10.1016/j.buildenv.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y.H., Lin J.Z. Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17(2):2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. Journal of Hospital Infection. 2006;64(2):100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y., Xu W., Gupta J.K., Guity A., Marmion P., Manning A. Experimental study on displacement and mixing ventilation systems for a patient ward. International Journal of HVAC&R Research. 2009;15(6):1175–1191. [Google Scholar]
- 6.Qian H., Li Y., Nielsen P.V., Hylgaard C.E. Dispersion of exhalation pollutants in a two-bed hospital ward with a downward ventilation system. Building and Environment. 2008;43(3):344–354. [Google Scholar]
- 7.Morawska L. Droplet fate in indoor environment, or can we prevent the spread of infection? Indoor Air. 2006;16(5):335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 8.Gupta J.K., Lin C.H., Chen Q. Flow dynamics and characterization of a cough. Indoor Air. 2009;19(6):517–525. doi: 10.1111/j.1600-0668.2009.00619.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S., Kato S., Yang J.H. Investigation into airborne characteristics of airflow due to coughing in a stagnant indoor environment. ASHRAE Transactions. 2006;112(1):123–133. [Google Scholar]
- 10.Melikov A., Kaczmarczyk J. Measurement and prediction of indoor air quality using a breathing thermal manikin. Indoor Air. 2007;17(1):50–59. doi: 10.1111/j.1600-0668.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun W., Ji J. Transport of droplets expelled by coughing in ventilated rooms. Indoor and Built Environment. 2007;16(6):493–504. [Google Scholar]
- 12.Bjørn E., Nielsen P.V. Dispersal of exhaled air and personal exposure in displacement ventilation rooms. Indoor Air. 2002;12(3):147–164. doi: 10.1034/j.1600-0668.2002.08126.x. [DOI] [PubMed] [Google Scholar]
- 13.Qian H., Li Y., Nielsen P.V., Hyldgaard C.E., Wong T.W., Chwang A.T.Y. Dispersion of exhaled droplet nuclei in a two-bed ward with three different ventilation systems. Indoor Air. 2006;16(2):111–128. doi: 10.1111/j.1600-0668.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 14.Chao C.Y.H., Wan M.P., Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M. Characterization of expiration air jets and droplet size distributions immediately at the mouth opening. Journal of Aerosol Science. 2009;40(2):122–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao C.Y.H., Wan M.P., Sze To G.N. Transport and removal of expiratory droplets in hospital ward environment. Aerosol Science and Technology. 2008;42(5):377–394. [Google Scholar]


