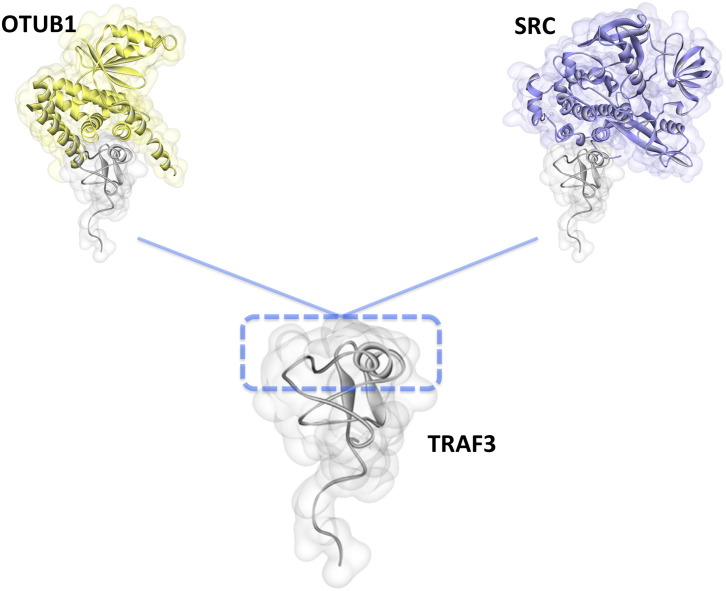
Fig. 8.
Modeled TRAF3 interactions through its RING domain. PRISM [36], [37], [38] predicted TRAF3 interactions with OTUB1 and SRC through its RING domain. These interactions have fully or partially overlapping interfaces, therefore they cannot co-exist. OTUB1 is a negative regulator of TRAF3-dependent IFN production [57]. However, SRC promotes TRAF3-dependent IFN production since its siRNA leads to decreased IFN production [61]. Competitive displacement of OTUB1 from TRAF3 may be the underlying cause for the increased IFN production in SRC recruitment to TRAF3, since TRAF3 signaling is not restricted to one negative regulator.