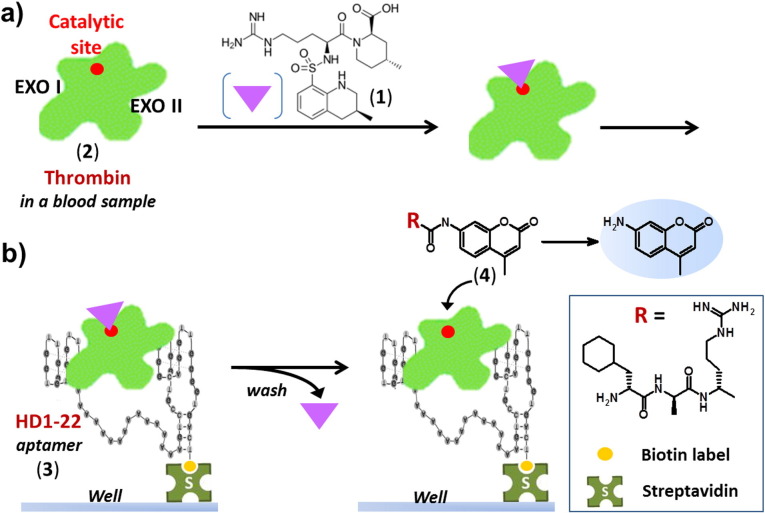
Fig. 10.
General principle of the oligonucleotide-based enzyme capture assay for thrombin detection developed by Pötzsch et al. [194]. a) During the blood sampling process, an anticoagulant buffer (citrate) containing the reversible active-site inhibitor argatroban (1) is added to the blood sample containing thrombin (2, PDB 1DWC). Complex formation between argatroban and thrombin efficiently prevents irreversible inhibition of thrombin by endogenous thrombin inhibitors. b) Wells of streptavidin-coated microtiter modules previously loaded with the 3′-biotinylated anti-thrombin aptamer HD1–22 (3) that simultaneously targets exosites I and II of thrombin are overlaid with plasma. After incubation and capturing of the argatroban-thrombin complex by HD1–22, wells are washed to remove plasma remains and reversibly bound argatroban. Subsequently, a thrombin-specific peptide substrate, bearing an AMC (7-amino-4-methylcoumarin) fluorogenic probe (H-D-CHA-Ala-Arg-AMC, 4), is added to quantitatively determine the amount of functional active thrombin captured in the wells.