Abstract
Objective We reported an outbreak of human parainfluenza virus type 1 (HPIV1) in a kindergarten and explored the genetic characteristics of HPIV1 hemagglutinin-neuraminidase (HN) and fusion (F) genes to provide more evidence about HPIV1 outbreaks.
Methods Suspected cases were the children with an influenza-like illness during June 20 to 26, 2018. Nasopharyngeal swabs were collected and screened to determine the presence of respiratory pathogens by real-time fluorescent quantitative polymerase chain reaction. The HN and F gene sequences of HPIV-positive samples were further amplified and sequenced to confirm the HPIV genotype and identify genetic characteristics. A phylogenetic tree, based on the HN and F genes, was reconstructed by maximum likelihood method.
Results Fourteen children in the outbreak were diagnosed as upper respiratory tract infection. The most common symptom was cough (10/14), followed by rhinorrhea (5/14), sore throat (4/14), headache (1/14), and abdominal pain (1/14). Eight patients were positive for HPIV1 and negative for other pathogens. Phylogenetic tree demonstrated that the eight strains from the year 2018 in our study located in the clade 2.3. Two specific substitutions (N333S and I509M) in the amino acids of the F protein and two substitutions (V19A and L436I) in the HN protein were different from other strains in the clade 2.
Conclusion HPIV1 was attributed to the outbreak, which may be related to the genetic variations of HPIV1.
Keywords: outbreak, parainfluenza virus, children
Introduction
Human parainfluenza viruses (HPIVs) are an important cause of respiratory illness in children and adults with a wide range of clinical manifestations, ranging from colds and croup to bronchiolitis and pneumonia, which cause a significant burden of disease in children and account for 40% of pediatric hospitalizations for lower respiratory tract illnesses and 75% of croup cases. 1 Population-based studies estimate that 0.39 to 3.01 per 1,000 children per year aged 0 to 4 years are infected with HPIV. 2
HPIV infections occur throughout the world with serotype-specific rates of infection which are determined by region, season, and population. Pan et al analyzed the epidemiological features of HPIV infection in 1,229 cases with severe acute respiratory infection in Beijing and found the highest yield rate in children under 5 years old. 3 Outbreaks of HPIV in household, nursing home, and daycare facilities have been described. 4 5 6 There have been several reports of HPIV3 outbreaks causing nosocomial infection 4 7 8 ; however, few outbreaks of HPIV1 have been reported. Therefore, Beijing Tongzhou Center for Disease Control and Prevention has continuously monitored HPIVs in this area, so as to permit timely finding of outbreaks, and thus prompt effective preventive and control measures.
In our study, we report one outbreak of HPIV1 in a kindergarten from Tongzhou District of Beijing, China, and demonstrate the genetic characteristics of HPIV1 in the outbreak to provide more evidence about HPIV1 outbreaks.
Methods
During June 2018, the outbreak occurred in a kindergarten from Tongzhou District of Beijing, China. The influenza-like illness (ILI) cases were included according to the WHO definition as sudden onset fever (>38°C) with cough or sore throat, in the absence of other diagnosis ( https://www.who.int/influenza/surveillance_monitoring/ili_sari_surveillance_case_definition/en/ ). To ensure complete patient inclusion, we also included the cases with temperature >37.5°C in this study. The research protocol was approved by Peking University Institutional Review Board (IRB00001052–19005).
The staff from the Institute for Infectious Diseases and Endemic Diseases Prevention and Control surveyed the kindergarten scene of the outbreak, interviewed teachers and parents, and acquired the records of diagnosis and treatment of pediatric patients. The basic information of the children in the study collected by staff included age, gender, diagnosis in the hospital, the onset and clinical course of the infection (including highest temperature, clinical manifestations, white blood cell counts), and the clinical outcome on the fourteenth day after disease onset.
Nasopharyngeal swabs were collected in the kindergarten by staff from Beijing Tongzhou Center for Diseases Prevention and Control and subjected for laboratory testing. RNA was extracted from each specimen by using QIAamp MinElute virus spin kits (Qiagen, Hilden, Germany). The cDNA sample was synthesized by using a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen). The samples were screened to determine the presence of the influenza virus (A and B), human metapneumovirus, human adenovirus, human bocavirus, enterovirus, respiratory syncytial virus (RSV), HPIV (types 1–4), coronaviruses (HKU1, NL63, 229E, and OC43), Legionella pneumophila, Haemophilusinfluenzae, Mycoplasma pneumoniae, Chlamydia pneumoniae, Bordetella pertussis, and Klebsiella pneumoniae by using molecular methods, as described previously. 9
The hemagglutinin-neuraminidase (HN) gene and fusion (F) gene sequences of HPIV-positive samples were further amplified and sequenced using nested PCR method to confirm the HPIV genotype and identify genetic characteristics, as described in a previous study. 10 The genomic sequences were assembled using Lasergene's DNA SeqMan software version 7.1.0 (DNA Star).
From GenBank, we obtained all available partial sequences of HPIV1, HN, and F genes. All comparison alignments were performed and a phylogenetic tree was reconstructed by maximum likelihood method with 1,000 bootstrap pseudo replicates using MEGA 7.0. Similarities between strains were calculated using BioEdit version 7.2.6 ( http://www.mbio.ncsu.edu/bioedit/bioedit.html ).
Results
Outbreak Identification
During June 2018, an outbreak of ILI occurred in 14 children in a kindergarten. This kindergarten had 9 classes in 3 grades, with 226 students and 51 staff members. It was a full-time teaching school with a canteen and without boarding students. The environment of the kindergarten was in good condition and no large-scale communal activities had been held recently. None of the children in the kindergarten were vaccinated against the influenza virus. All the pediatric patients were from one primary class, which had 27 students (13 boys and 14 girls) and one head teacher.
The index pediatric patient was a 4-year-old boy. He had fever on June 20, 2018 with the highest temperature of 39.5°C. He also had the symptoms of cough, sore throat, and rhinorrhea. He went to hospital at the same day and was diagnosed with otitis media and upper respiratory tract infection (URTI). His guardian denied that there was any recent exposure history to fever cases or sick/dead poultry.
The onset times of the pediatric patients were between June 20 and 26, 2018 ( Supplementary Fig. S1 , available online only). Twelve of 14 pediatric patients were seen face-to-face in the kindergarten and throat swabs were collected. The other two pediatric patients were surveyed by the telephone. All the pediatric patients went to hospital at the day of disease onset and 12 pediatric patients were diagnosed with URTI and 1 was diagnosed as URTI with tonsillitis. Detailed information about the pediatric patients is shown in Table 1 . There were six boys and eight girls. All the patients had a maximum temperature of ≥37.5°C and 10 had a fever of ≥38.0°C. The most common symptoms were cough (10/14), followed by rhinorrhea (5/14), sore throat (4/14), headache (1/14), and abdominal pain (1/14). Among the 10 patients who had full blood counts performed, the white blood cell count was normal (4–10 × 10 9 /L) in five, high (≥10 × 10 9 /L) in three, and low (<4 × 10 9 /L) in two. All the patients had good prognosis without hospitalization, and no severe or fatal patients occurred.
Table 1. Detailed information on the children affected by the outbreak during June 2018, Beijing, China.
| S.No | Sex | Age (y) | Diagnosis | Onset date (in 2018) | Highest temperature (°C) | Cough | Pharyngalgia | Rhinorrhea | Headache | Abdominal pain | WBC (×10 9 /L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 4 | Otitis media, URTI | June 20 | 39.5 | Yes | Yes | Yes | No | No | 3.51 |
| 2 | Female | 4 | URTI | June 22 | 38.3 | Yes | No | No | No | No | – |
| 3 | Male | 4 | URTI | June 22 | 40 | Yes | Yes | Yes | No | No | 6.8 |
| 4 | Male | 4 | URTI | June 22 | 40 | Yes | No | No | Yes | No | 3.98 |
| 5 | Male | 4 | URTI | June 23 | 39 | Yes | No | No | No | No | 7.5 |
| 6 | Female | 4 | URTI | June 23 | 38.8 | Yes | Yes | No | No | No | – |
| 7 | Female | 4 | Tonsillitis, URTI | June 23 | 39.1 | Yes | No | No | No | Yes | 10.67 |
| 8 | Female | 4 | URTI | June 24 | 38 | Yes | No | Yes | No | No | – |
| 9 | Female | 4 | URTI | June 22 | 39 | Yes | No | Yes | No | No | 12.19 |
| 10 | Female | 4 | URTI | June 22 | 38.5 | Yes | Yes | No | No | No | 10 |
| 11 | Female | 4 | URTI | June 23 | 38.5 | Yes | No | Yes | No | No | 6.51 |
| 12 | Male | 4 | URTI | June 23 | 38.3 | Yes | No | No | No | No | 5.97 |
| 13 | Male | 4 | URTI | June 26 | 37.7 | Yes | No | No | No | No | – |
| 14 | Female | 4 | URTI | June 26 | 37.8 | Yes | No | No | No | No | 8.14 |
Abbreviations: URTI, upper respiratory tract infection; WBC, white blood cell.
Twelve nasopharyngeal swabs were collected and sent to the laboratory for detection. The results of RT-PCR showed that eight patients were positive for HPIV1 and negative for other pathogens. The other four patients, including the index case, were negative for all pathogens.
HN and F Genes of HPIV1
In the study, the HN and F gene sequences of eight HPIV1 strains were amplified and sequenced. A phylogenetic tree of HPIV1 based on the complete F gene was constructed using maximum likelihood method with the MEGA 7.0 ( Fig. 1 ). Three clusters were formed in the phylogenetic tree. Clade 1 included strains from United States during 1997 to 1999. Clade 3 included strains after the year 2009. Clade 2 included the most strains during 1999 to 2009 (clade 2.1 and clade 2.2) and a few strains after 2009 (clade 2.3). The eight strains from the year 2018 in our study located in the clade 2.3. The identities of nucleotide sequences were 98.1 to 98.7% and the identities of amino acid sequences were 99.0 to 99.8% among the clade 2.3. When adding the strains from Japan ( Supplementary Fig. S2 , available online only), the LC076588/Japan/2013 located in clade 2.3, which was close to the strains in our study and the other Japan strains located in clade 3. We observed two specific substitutions (N333S and I509M) in the amino acids of the F protein, which was different from other strains in the clade 2 ( Supplementary Fig. S3 , available online only).
Fig. 1.
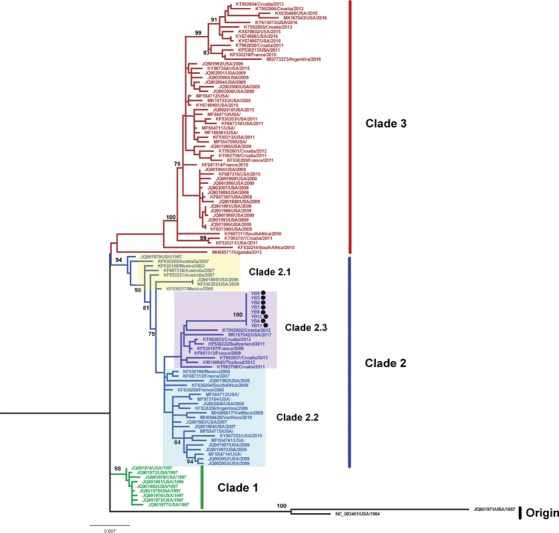
Phylogenetic analysis of HPIV1 based on F gene constructed using a 2030 nucleotide sequence corresponding to nucleotide 4813–6842 in the Washington_1964 strain from the USA (GenBank accession no: NC_003461). A tree with 1,000 bootstrap replicates was reconstructed using maximum likelihood method with the MEGA 7.0 based on nucleotide sequences. The location and year of collection are shown. The strains detected in this study are in black solid circle.
The phylogenetic tree based on the complete HN gene also formed three clades ( Fig. 2 ), similar to the F phylogenetic tree. The eight strains from the year 2018 in our study located in the clade 2.3. The identities of nucleotide sequences were 98.2 to 99.1% and the identities of amino acid sequences were 99.3 to 99.4% among the clade 2.3. The HN gene sequences revealed spatial clustering feature in the internal clades ( Fig. 2 and Supplementary Fig. S4 , available online only). We observed two specific substitutions (V19A and L436I) in the clade 2.3 of the amino acids of the HN protein, which was different from other clades in the clade 2 ( Supplementary Fig. S5 , available online only). Two specific substitutions (K1T and L108F) were only detected in our strains.
Fig. 2.
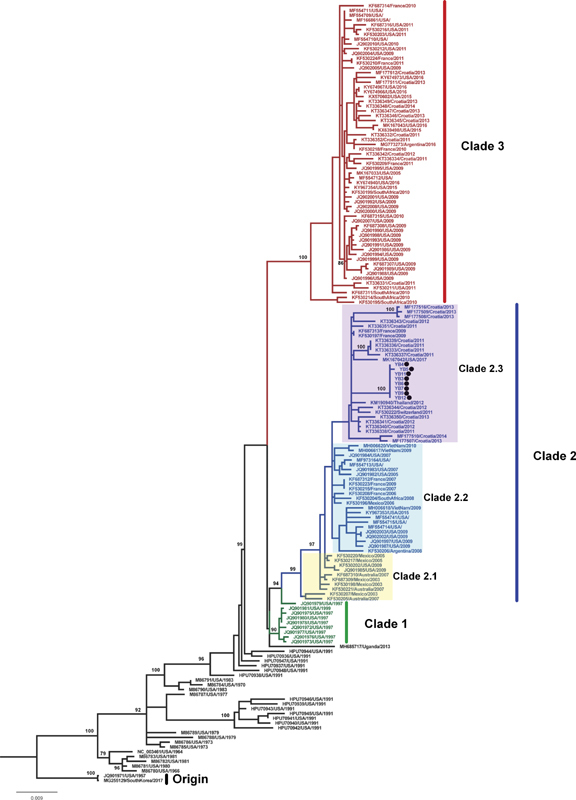
Phylogenetic analysis of HPIV1 based on HN gene constructed using a 1894 nucleotide sequence corresponding to nucleotide 6847–8740 in the Washington_1964 strain from the USA (GenBank accession no: NC_003461). A tree with 1,000 bootstrap replicates was reconstructed using maximum likelihood method with the MEGA 7.0 based on nucleotide sequences. The location and year of collection are shown. The strains detected in this study are in black solid circle.
Control of the Outbreak
After the samples were sent to laboratory for testing, all the children with fever and respiratory symptoms were required to be isolated at home for 7 days. The kindergarten implemented the regular morning and afternoon inspection mechanism to monitor the absenteeism registration system and follow-up patients. The classrooms were conducted with disinfection and ventilation. The class with the outbreak of ILI cases suspended classes for 1 week till no new ILI cases occurred in the next week. All the pediatric patients recovered and resumed classes.
Discussion
In this study, we reported an outbreak of HPIV1 infection causing URTI in a kindergarten, which has rarely been reported previously. Four specific substitutions in the amino acids of HN and F were detected.
The 12 children in the outbreak manifested typical influenza-like symptoms. HPIV can especially cause URTI and severe lower respiratory tract diseases. 11 HPIV1 is the major cause of laryngotracheobronchitis and croup in children. 12 The majority of infections occur in the children aged 7 to 36 months, with a peak incidence observed in the second and third year of life. 13 Due to the long period post-HPIV1 infection, 14 the index pediatric patient was in convalescence and tested negative for HPIV1. It is worth noting that the index pediatric patient also had a diagnosis of otitis media, which is also one of the typical features of the infection. 1 We also paid attention to the diseases cause by HPIV1 beyond respiratory tract diseases.
Several studies have documented distinct temporal trends for HPIV serotypes 14 ; however, these vary from region to region. HPIV1 occurs mostly in summer in Spain. 15 In southern China, seasonal peaks of HPIV1 occur in late summer and early autumn. 16 In Beijing, HPIV1 had a higher frequency in odd-numbered years, and the peak was in summer. 17 This outbreak occurred in the summer that is in line with the trend of high incidence. The high-occurrence season offers the possibility of outbreak. Therefore, in the summer, the cause of the outbreak of ILI cases should be taken HPIV1 into consideration by pediatrician.
HPIV possess two major structural proteins, which are essential for virus assembly: HN and F. 14 The functions of HN, found on the lipid envelope of HPIV and infected cells, is in virus-host cell attachment via sialic acid receptors; the F protein that allows the viral nucleocapsid to enter and infect a host cell, is required for membrane fusion between host cells. We observed two specific substitutions in the amino acids of the F protein and HN protein, respectively, which revealed genetic variability. 18 This strain caused the outbreak that may be related to the genetic variations of the strain from other strains. Whether the outbreak is associated with the four specific substitutions at these sites requires further study. From the phylogenetic tree of HPIV1 based on the complete HN and F gene, the HPIV1 sequences revealed the temporal characteristic. Meanwhile, the spatial spread may lead to the disorder in the temporal clusters, such as the eight strains in 2018 from our study located in the clade 2.3. In the same cluster, the sequences also revealed the spatial characteristic. However, we did not get the strains from China in GenBank. More sequences from China are needed to describe the genetic and evolutional characteristics of HPIV1.
HPIV1 was attributed to the outbreak, which may be related to the genetic variations of HPIV1.This study provides evidence that HPIV1 can cause outbreaks and contributes to literature on the clinical manifestation and genetic characteristics of HPIV1.
Funding Statement
Funding The study was supported by China Mega-Project for Infectious Diseases Grant (2017ZX10103004), National Natural Science Foundation of China (81703274), and the Fundamental Research Funds for the Central Universities (BMU20170607) and Peking University Medicine Seed Fund for Interdisciplinary Research (BMU2018MX009). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest None declared.
These authors contributed equally to this work.
Ethical Approval
This study was performed with the approval of the Ethical Committees of Peking University Institutional Review Board. The methods were performed according to approved guidelines.
Availability of Data and Materials
The dataset used and analyzed during this study are available from the corresponding author on reasonable request.
Supplementary Material
References
- 1.Branche A R, Falsey A R. Parainfluenza virus infection. Semin Respir Crit Care Med. 2016;37(04):538–554. doi: 10.1055/s-0036-1584798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg G A, Hall C B, Iwane M K et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(05):694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Pan Y, Zhang Y, Shi W et al. Human parainfluenza virus infection in severe acute respiratory infection cases in Beijing, 2014-2016: A molecular epidemiological study. Influenza Other Respir Viruses. 2017;11(06):564–568. doi: 10.1111/irv.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan S, Gillespie E, Stuart R L. A parainfluenza virus type 3 outbreak at a residential aged care facility: The role of microbiologic testing in early identification and antimicrobial stewardship. Am J Infect Control. 2017;45(02):203–205. doi: 10.1016/j.ajic.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow K W, Tamblyn S E, Blair G. A respiratory outbreak due to parainfluenza virus type 3 in a home for the aged--Ontario. Can Commun Dis Rep. 1995;21(07):57–61. [PubMed] [Google Scholar]
- 6.Ni Z-M, Huang S-W, Xu D-G. Investigation of an outbreak of parainfluenza virus infection. Zhejiang Preventive Medicine. 2014;26(11):1141–1142. [Google Scholar]
- 7.Harvala H, Gaunt E, McIntyre C et al. Epidemiology and clinical characteristics of parainfluenza virus 3 outbreak in a Haemato-oncology unit. J Infect. 2012;65(03):246–254. doi: 10.1016/j.jinf.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Kim T, Jin C E, Sung H et al. Molecular epidemiology and environmental contamination during an outbreak of parainfluenza virus 3 in a haematology ward. J Hosp Infect. 2017;97(04):403–413. doi: 10.1016/j.jhin.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren L, Gonzalez R, Wang Z et al. Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-2007. Clin Microbiol Infect. 2009;15(12):1146–1153. doi: 10.1111/j.1469-0691.2009.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Košutić-Gulija T, Slovic A, Ljubin-Sternak S, Mlinarić-Galinović G, Forčić D. A study of genetic variability of human parainfluenza virus type 1 in Croatia, 2011-2014. J Med Microbiol. 2016;65(08):793–803. doi: 10.1099/jmm.0.000297. [DOI] [PubMed] [Google Scholar]
- 11.Xiao N G, Duan Z J, Xie Z P et al. Human parainfluenza virus types 1-4 in hospitalized children with acute lower respiratory infections in China. J Med Virol. 2016;88(12):2085–2091. doi: 10.1002/jmv.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima K, Takahashi T, Ito S et al. Terminal sialic acid linkages determine different cell infectivities of human parainfluenza virus type 1 and type 3. Virology. 2014;464-465:424–431. doi: 10.1016/j.virol.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 13.Marx A, Török T J, Holman R C, Clarke M J, Anderson L J. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997;176(06):1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 14.Pawełczyk M, Kowalski M L. The role of human parainfluenza virus infections in the immunopathology of the respiratory tract. Curr Allergy Asthma Rep. 2017;17(03):16. doi: 10.1007/s11882-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Álvarez-Argüelles M E, Rojo-Alba S, Pérez Martínez Z et al. New clinical and seasonal evidence of infections by human parainfluenza virus. Eur J Clin Microbiol Infect Dis. 2018;37(11):2211–2217. doi: 10.1007/s10096-018-3363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W K, Liu Q, Chen D H et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis. 2013;13(01):28. doi: 10.1186/1471-2334-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Zhao L Q, Zhu R N et al. Parainfluenza Virus Types 1, 2, and 3 in pediatric patients with acute respiratory infections in Beijing during 2004 to 2012. Chin Med J (Engl) 2015;128(20):2726–2730. doi: 10.4103/0366-6999.167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henrickson K J. Parainfluenza viruses. Clin Microbiol Rev. 2003;16(02):242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and analyzed during this study are available from the corresponding author on reasonable request.


