Abstract
Porcine epidemic diarrhea virus (PEDV) causes acute diarrhea, vomiting, dehydration, weight loss, and high mortality rate in neonatal piglets. Porcine epidemic diarrhea (PED) has been reported in Europe, America, and Asia including Thailand. The disease causes substantial losses to the swine industry in many countries. Presently, there is no effective PEDV vaccine available. In this study, we developed a plant-produced monoclonal antibody (mAb) 2C10 as a prophylactic candidate to prevent the PEDV infection. Recently, plant expression systems have gained interest as an alternative for the production of antibodies because of many advantages, such as low production cost, lack of human and animal pathogen, large scalability, etc. The 2C10 mAb was transiently expressed in Nicotiana benthamiana and lettuce using geminiviral vector. After purification by protein A affinity chromatography, the antibody was tested for the binding and neutralizing activity against PEDV. Our result showed that the plant produced 2C10 mAb can bind to the virus and also inhibit PEDV infection in vitro . These results show excellent potential for a plant-expressed 2C10 as a PEDV prophylaxis and a diagnostic for PEDV infection.
Key words: porcine epidemic diarrhea virus (PEDV), 2C10 monoclonal antibody (mAb), molecular farming, Nicotiana benthamiana, Solanaceae, Lactuca sativa, Asteraceae
Introduction
Porcine epidemic diarrhea (PED) is an enteric disease that causes the significant loss for the swine industry. The disease first occurred in Europe in the early 1970s and the virus was first isolated in Belgium in 1978 1 . Subsequently, the disease was also reported in the United States 2 and several countries in Asia, including China 3 , Korea 4 , Taiwan 5 , Japan 6 , and Thailand 7 . PED is characterized by intense diarrhea, vomiting, weight loss, and dehydration. These symptoms result in the death of newborn piglets and weight loss in all pig ages, which adversely affects growth performance of growing pigs 1 , 8 . PED diagnosis cannot be made purely on the basis of clinical signs and histopathological lesions. Therefore, the diagnosis to confirm the presence of porcine epidemic diarrhea virus (PEDV) or its antigens must be conducted in the laboratory.
PEDV is an enveloped virus with a single-stranded positive-sense RNA and belongs to the Alphacoronavirus genus in the Coronaviridae family (ICTV, 2012). Its genome is approximately 28 kb encoding seven viral proteins: ORF1A, ORF1B, spike (S), OFR3, small envelope protein (E), membrane (M), and nucleocapsid (N) 9 . Among these viral proteins, S- protein is the key protein responsible for the viral entry into the host cell. It is the envelope type I glycoprotein that can bind to the receptor on the host cell, subsequently fuse the viral membrane to the host membrane, and then release the nucleocapsid protein into the host cell 10 . Vaccination with the recombinant S-protein was shown to protect the piglets against PEDV infection 11 . Thus, S-protein is one of the targets for PEDV vaccine development.
There are several neutralizing epitopes on the S-protein 12 , 13 , 14 . Among these epitopes, 2C10 is one of the peptides that are recognized by neutralizing monoclonal antibody (mAb) against PEDV 15 . The recombinant 2C10 single-chain antibody, consisting of variable regions of heavy (VH) and light chains (VL), was previously expressed in Escherichia coli and also showed the PEDV neutralization 16 . This can be developed to use as PEDV diagnostic and prophylaxis treatment.
The goal of this study was to develop 2C10 mAb in plants. Recently, plants have been used as factories to effectively produce several recombinant proteins because of several advantages over other protein expression systems, including low manufacturing cost, high protein expression level, scalability, and lack of human and animal pathogen 17 , 18 . Moreover, plants contain the post-translational modification, which are often critical to the proper protein functions 19 , 20 . The two main systems for producing proteins in plants are stable transgenic expression and transient expression. The establishment of transgenic plants is a time-consuming process with limitation of relatively low protein expression level. For transient expression, plant viral vectors were developed to increase the protein expression level 21 . Among different viral vectors, geminiviral vector was developed to produce several proteins in plants such as antigens and antibodies 22 , 23 , 24 .
In this study, we used geminiviral vectors to produce 2C10 mAb in Nicotiana benthamiana Domin. (Solanaceae) and Lactuca sativa L. var. longifolia (Asteraceae) by transient expression. The seeds of N. benthamiana were provided from Julian Maʼs Lab (London, UK). N. benthamiana is the most common host plant for transient expression because of its high biomass yield. Lettuce is another rapid-growing plant that also produces high biomass and contains lower amount of phenolics and alkaloids, compared to tobacco plants. This is the advantage for the protein purification downstream process 24 . Lettuce was obtained commercially from a local grocery store with romaine lettuce labeled on the package. We tested the 2C10 mAb expressed in N. benthamiana and lettuce for PEDV binding and neutralizing activity. This plant-produced mAb can be applied to use as diagnostic kit or prophylactic treatment for PEDV infection, which will be very useful for the swine industry.
Results
The genes optimized for plant codon usage encoding the VL and VH chains of 2C10 ( Fig. 1 ) were amplified and fused with constant regions of IgG1 mAb 25 . The fused genes were inserted into geminiviral replicon vector (pBY030.2R) ( Fig. 2 ) and introduced to Agrobacterium tumefaciens strain GV3101. The 2C10 mAb was expressed in N. benthamiana by co-infiltration with the mixture of A. tumefaciens containing pBY2C10-kappa, pBY2C10-kamma, and p19. The p19 used in this study was the gene silencing inhibitor p19 from tomato bushy stunt virus using the non-replicating expression vector. At day 5 post-infiltration, the 2C10 mAb in plant extract was detected by western blot analysis using mouse anti-human IgG antiserum, Fc specific and mouse anti-kappa light chain antiserum. The result showed that 2C10 IgG was produced in N. benthamiana , containing both heavy chain and light chain at approximately 50 and 25 kDa in reducing condition, respectively ( Fig. 3 A ). The assembly of 2C10 IgG was determined after protein A affinity purification by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The 2C10 mAb purified from plant proteins were analyzed under reducing and non-reducing conditions. Under reducing condition, two bands of heavy and light chains were detected at 50 kDa and 25 kDa, respectively ( Fig. 3 B ), while the purified protein had the band at 150 kDa ( Fig. 3 B ), which confirms the assembly of the whole IgG molecule.
Fig. 1.
Optimized nucleotide and deduced amino acid sequence of 2C10 VL ( A ) and VH ( B ).
Fig. 2.
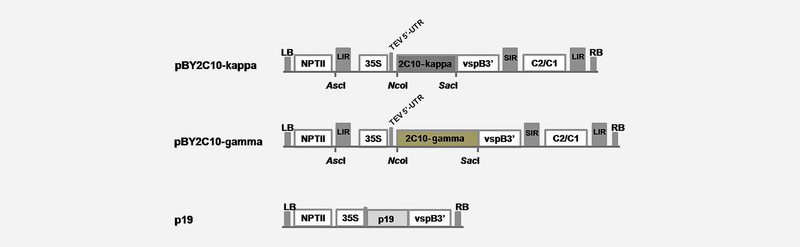
Schematic representation of plant expression vectors used in this study. 35S/TEV5′: CaMV 35S promoter with tobacco etch virus 5′ UTR; VSP 3′: soybean vspB gene 3′ element; NPTII: expression cassette encoding nptII gene for kanamycin resistance; LIR: long intergenic region of BeYDV genome; SIR: short intergenic region of BeYDV genome; C2/C1: BeYDV ORFs C1 and C2 that encode for replication initiation protein (Rep) and RepA; RB: the right border of the T-DNA region; LB: the left border of the T-DNA region.
Fig. 3.
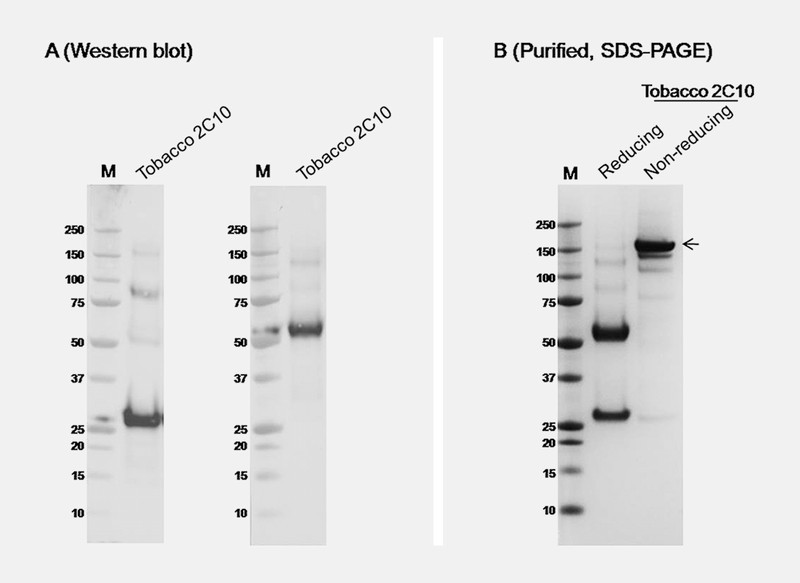
Expression and purification of 2C10 mAb in N. benthamiana . Western blot analysis ( A ) of 2C10 mAb in crude leaf extracts 5 d after expression vectors were co-infiltrated is detected by anti-human kappa and anti-human IgG Fc specific (gamma). SDS-PAGE of purified 2C10 mAb ( B ) shows in reducing and non-reducing condition. M: protein marker. Arrow indicates fully assembled IgG.
To test the effectiveness of geminiviral vector in producing the 2C10 mAb in lettuce, we investigated the transient expression of 2C10 mAb in romaine lettuce from the local store. Agrobacterium cultures using for lettuce were similar to the experiment above. At day 4 post- infiltration, western blot analysis of infiltrated lettuce leaves confirmed the expression of both heavy chain and light chain of 2C10 IgG at the expected size ( Fig. 4 A ), similar to the 2C10 expressed in N. benthamiana ( Fig. 3 A ). The 2C10 mAb was purified from the lettuce proteins with protein A affinity chromatography ( Fig. 4 B ).
Fig. 4.
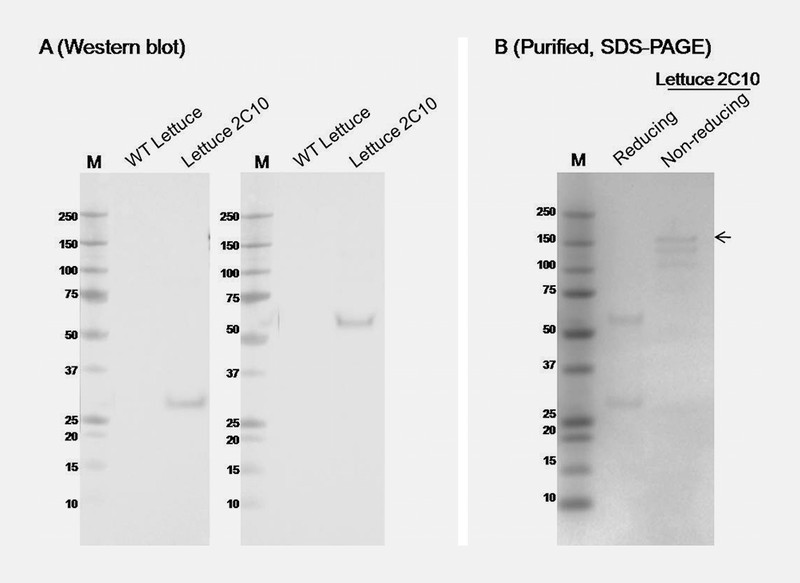
Expression and purification of 2C10 mAb in lettuce. Western blot analysis ( A ) of 2C10 mAb in crude leaf extracts 5 d after expression vectors were co-infiltrated is detected by anti-human kappa and anti-human IgG Fc specific (gamma). SDS-PAGE of purified 2C10 mAb ( B ) shows in reducing and non-reducing condition. M: protein marker. Arrow indicates fully assembled IgG.
The PEDV binding ability of plant produced 2C10 mAb was tested by indirect ELISA (enzyme-link immunosorbent assay). Plant-produced 2C10 IgG from tobacco and lettuce was diluted 1 : 1000 before incubated in wells containing immobilized PEDV. Detection with HRP (horseradish peroxidase) labeled anti-human IgG antiserum yielded OD450 measurement. The results showed that 2C10 mAb produced in tobacco and lettuce could bind to PEDV approximately 1.7 and 1.6 times more than the negative control ( Fig. 5 ). However, glutathione S -transferase (GST) fused with truncated S-protein of PEDV showed no binding to PEDV; the binding ratio to PEDV is approximately 1 compared to negative control. These results suggested that the plant-produced 2C10 mAb might be developed for use as a diagnostic for PEDV infection.
Fig. 5.
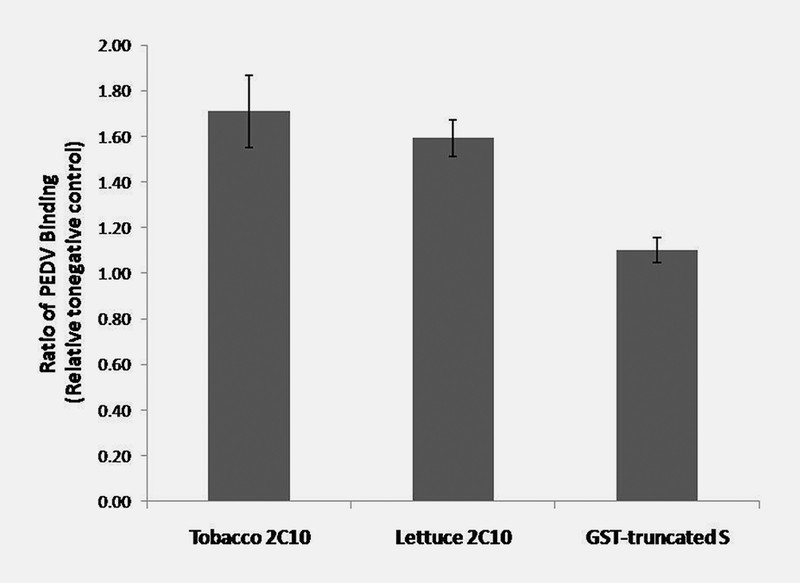
The binding efficiency of plant 2C10 mAbs to PEDV. Plant-produced 2C10 IgG from N. benthamiana and lettuce were diluted 1 : 1000 before incubated in wells containing immobilized PEDV. Detection with HRP-labeled anti-human IgG antiserum yielded OD450 measurements. Data are means of the ratio of the binding of plant produced 2C10 or GST-truncated S to media of Vero cells without virus ± standard deviation (SD) of samples from three independent experiments. GST-truncated S: glutathione S -transferase fused with truncated spike protein of PEDV.
The 2C10 mAb showed some binding activity to PEDV ( Fig. 5 ). To evaluate the neutralization potential of 2C10 mAb, the PEDV neutralization assay was performed using 2C10 produced from N. benthamiana . PEDV virus (100 – 300 TCID50/50 µL) was incubated with the 2C10 mAb for 1 h at 37 °C. The antibody-PEDV mixture was added to Vero cell and incubated for 1 h at 37 °C. The result showed that 1 : 32 dilution ratio of 2C10 mAb was able to neutralize PEDV infection in Vero cell ( Table 1 ) because there is no cytopathic effect. However, after the antibody was diluted to 1 : 64, the cytopathic effect was shown. Thus, plant-produced 2C10 mAb retained neutralizing activity against infectious PEDV, which suggested the potential to use as PEDV prophylaxis treatment.
Table 1 Result of viral neutralization assay. Different dilutions of plant-produced 2C10 IgG were incubated with PEDV 1 h before transfer to Vero cells. After 1 h, the mixed of viral and 2C10 IgG were removed. The Vero cells plate was continuous incubated at 37 °C for 5 – 7 days for CPE determination. Positive control: colostrum sample with PEDV neutralizing antibody; negative control: colostrum sample without PEDV neutralizing activity; +: cytopathic effect; −: no cytopathic effect.
| mAb/ dilution | 1 : 4 | 1 : 8 | 1 : 16 | 1 : 32 | 1 : 64 | 1 : 128 | 1 : 256 |
|---|---|---|---|---|---|---|---|
| N. benthamiana 2C10 | – | – | – | – | + | + | + |
| 1× PBS | + | + | + | + | + | + | + |
| Negative control | + | + | + | + | + | + | + |
| Positive control | – | – | – | – | – | – | – |
Discussion
PED is a major reason for the economic loss in swine industry 26 , 27 . PEDV infection induces death in neonatal pigs due to watery diarrhea and dehydration. Previous study reported that there are over one million piglet deaths from PEDV infection during the outbreak in China in 2010, which created large economic losses 28 . For PEDV prevention, there are different types of PEDV vaccine commercially, including live viral vaccine and inactivated virus 29 , 30 . However, these vaccines did not significantly reduce the morbidity rate due to diarrhea, because several factors including route of administration and strains of vaccine virus affected the poor lactogenic immunogenicity of these commercial vaccines 31 . Another potential approach to protection of neonatal piglets is passive immunity induced by effective quantities of protective antibodies obtained from sow colostrum and milk. Artificial passive immunization by oral administration of specific antibodies represents an attractive approach against gastrointestinal pathogens such as PEDV 26 . Several PEDV neutralizing antibodies were developed previously 15 , 16 , 32 , 33 , and they might be good candidates for use as PEDV prophylactic agents. However, an effective antibody production system with low production cost is required for the animal industry.
Plant-based expression is becoming attractive for recombinant protein production. Several conventional systems, such as E. coli , yeast, mammalian cells, insect cells, etc., are available commercially. Among these production platforms, plants have several advantages over other systems, including low production cost, large scalability, lack of human and animal pathogen, and post-translational modification, which affect the function of many recombinant glycoproteins. In the present study, tobacco ( N. benthamiana ) and lettuce ( L. sativa ) were used to produce 2C10 mAb, which previously showed the PEDV neutralization 16 .
Among all PEDV neutralizing mAb, 2C10 is one of a good candidate for using to inhibit PEDV infection 15 , 32 , 34 , 35 , 36 , 37 , 38 . The 2C10 mAb recognizes GPRLQPY motif found on carboxy-terminal region of PEDV S-protein 12 . Moreover, this mAb also showed strong binding to the peptides SHRLP(Y/Q)(P/V) or GPRPVTH on the g3p minor coat protein and strong neutralizing activity against KPED-9 strain 15 .
The nucleotide sequences of 2C10 VH and VL from previous work 24 were codon usage optimized for N. benthamiana and synthesized. After fusing both VH and VL to the constant region of heavy chain (CH) and light chain (CL), the 2C10 gamma and kappa gene were cloned into geminiviral replicon vector, separately. To test whether 2C10 mAb produced from plants can function, we used the human IgG constant region which is available in the lab. If we get the promising result, the porcine IgG constant region will be used for further study. Geminiviral replicon systems have been used for production of several recombinant proteins in plants, such as vaccine antigens 22 , 23 , 24 , 39 , 40 and antibodies 22 , 24 , 25 . The transient expression in plants using geminiviral vectors can rapidly produce high level of recombinant protein expression.
In this study, 2C10 mAb was transiently expressed in tobacco and lettuce on day 5 and day 4 after agroinfiltration, respectively ( Fig. 3 A and Fig. 4 A ). This expression process generated 2C10 mAb in a short period of time. After purification process, the assembly of full-size 2C10 IgG (150 kDa) produced from both plants was detected in SDS-PAGE under non-reducing condition ( Fig. 3 B and Fig. 4 B ). The quantity of corrected IgG was found in tobacco more than lettuce. However, the antibodies produced from both plants clearly showed binding to PEDV in vitro ( Fig. 5 ). Our result showed that the binding of plant-produced 2C10 mAb to PEDV increased approximately 1.7 times. However, the binding of another protein, GST-truncated S, is similar to negative control (the binding ratio is approximately 1). The binding activity of plant-produced 2C10 mAb strongly suggests that it can be developed to use as a reagent in the assays for detection of PEDV.
To test the neutralization of PEDV by 2C10, PEDV strain ST was used in this study. The 2C10 IgG produced from N. benthamiana showed the neutralizing activity at dilution 1 : 32 ( Table 1 ). In this study, the neutralizing activity of plant-produced 2C10 was not strong compared to the neutralizing activity of 2C10 mAb against KPED-9 strain in previous study 15 . The level of neutralizing activity might be different depending on the strain of the virus. This data confirmed that the plant-produced 2C10 mAb neutralized PEDV infection in vitro , suggesting the potential to use as PEDV prophylactic agent. Previous data reported that protecting suckling piglets from PEDV infection also depends on the secretory IgA (sIgA) antibodies in milk 41 . The sIgA is more resistant to protease than IgG, which is suitable to use as the oral vaccine 42 . Therefore sIgA is a good approach to use as a prophylactic and therapeutic treatment. Plants were shown to be an effective platform for sIgA production 43 , 44 , 45 . Thus, we can develop this 2C10 mAb to produce sIgA from plants in the future, which will be more effective PEDV prophylactic.
The results of the present study provided a proof of concept in which the PEDV specific antibody can be produced through plant expression system and the produced antibody has an in vitro efficacy against PEDV. Further in vivo investigation involving oral application of plant produce antibody in piglets against oral PEDV challenge is suggested. The in vivo study, although can address the efficacy of the produced antibody, is needed to control several factors including methods to prevent enzymatic activities in gastrointestinal tracts, pH factors, and delivery system in which is beyond the scope of the study. The level of antibody needed to protect young pigs from the PEDV infection is another study that is needed for further investigation. In this study, the neutralization showed at titer 1 : 32 and at higher concentration of the antibody. However, at which certain level is beyond the scope of the study as the level of antibody induced by either natural infection or vaccination that provide a complete protection is presently yet to be known.
In conclusion, we developed a novel plant-produced PEDV 2C10 mAb, which showed binding and neutralizing activity against PEDV. This technology provides an effective and low-cost platform to produce PEDV mAb, which could be developed for PEDV diagnostic and prophylactic agents. The platform developed here will be beneficial, especially to the swine industry.
Materials and Methods
Design of the constructs for producing 2C10 mAb in plants
We designed plant-optimized DNA sequences encoding 2C10 VH and VL based upon previous study 16 , using codons that are preferred in tobacco. The VH was fused to the N-terminus of a gene encoding constant region of the humanized 62-71-3 mAb 46 . The synthetic VH gene was amplified using primers NcoI-VH-F and PmlI-VH-R whereas the 62-71-3 CH was amplified using PmlI-CH-F and SacI-CH-R. All primer sequences are in Table 2 . The overlapped extension PCR between VH and CH was performed using primers NcoI-VH-F and SacI-CH-R. For the cloning of 2C10 light chain, the synthetic VL gene was amplified using primers NcoI-VL-F and XhoI-VL-R whereas the 62-71-3 CL was amplified using primers XhoI-CL-F and SacI-CL-R. The overlapped PCR between VL and CL was performed using primers NcoI-VL-F and SacI-CL-R. The genes of 2C10 heavy and light chain were inserted into the geminiviral vector via NcoI and SacI sites. Two geminiviral vectors, 2C10 heavy chain and light chain genes, was co-infiltrating with p19 vector.
Table 2 Primer list.
| Primer Name | Sequencing (5′- 3′) |
|---|---|
| NcoI-VL-F | CCATGGACATGAGAGTTCCAGCGATATTG |
| XhoI-VL-R | CTCGAGACCCCCTTGATCTCC |
| XhoI-CL-F | CTCGAGGACTGTTGCTGCTCC |
| SacI-CL-R | GAGCTCTTAGCACTCGCCCCTATTG |
| NcoI-VH-F | CCATGGAACTTGGACTTTCTTGG |
| PmlI-VH-R | CACGTGTGAGTCTTATCGCAG |
| PmlI-CH-F | CACGTGTCCACCATGTCCAGC |
| SacI-CH-R | GAGCTCTTACTTGCCAGGGGACAAAG |
Plant inoculation and protein expression
The seeds of N. benthamiana were provided from Julian Maʼs Lab (London, UK). N. benthamiana plants were co-infiltrated with Agrobacterium GV3101 strains containing pBY2C10-gamma, pBY2C10-kappa, and p19 that protects gene silencing of transient protein production in plant ( Fig. 2 ). The final OD 600 of mixed Agrobacterium in equal amount of the three strains for vacuum infiltration is 0.25. Plants were maintained in the growth chamber for 5 d before protein extraction. Romaine lettuce ( L. sativa ) was obtained commercially from a local grocery store. The agroinfiltration of lettuce was performed as previously described 24 . After vacuum infiltration with Agrobacterium , lettuce heads were covered with Saran wrap and kept in the growth chamber for 4 d before protein extraction.
Protein purification
Protein purification Infiltrated tobacco and lettuce leaves were homogenized by using a blender with 1× phosphate-buffered saline (1× PBS: 137 mM NaCl; 2.7 mM KCl; 4.3 mM Na 2 HPO 4 ; 1.47 mM KH 2 PO 4 at pH 7.4), pH 7.5. Crude extract was filtered through Miracloth and centrifuged at 26,000 g for 20 min. The solution was filtered with 0.2 µm filter before loading into the protein A bead column. After washing the column with PBS, pH 7.5, for 10 bed volumes, the protein was eluted with 100 mM glycine, pH 2.5. After the protein was eluted from the column, 1 M Tris base was added to neutralize to a final pH of 7.5.
SDS-PAGE and western blotting
The purified plant 2C10 mAb protein was mixed with SDS loading sample buffer (250 mM Tris-HCl pH 6.8, 10% [w/v] SDS, 30% [v/v] glycerol, 5% [v/v] β -mercaptoethanol, and 0.02% [w/v] bromophenol blue) for reducing SDS-PAGE or SDS loading sample buffer without β -mercaptoethanol for non-reducing SDS-PAGE and boiled for 5 min. Proteins were separated using 10% SDS-PAGE. Proteins were stained with Coomassie Brilliant Blue G-250. For western blot, proteins were transferred to a nitrocellulose membrane (Bio-Rad) and probed with HRP-conjugated mouse anti-human IgG1 antiserum or Fc-specific or HRP-conjugated mouse anti-kappa light chain antiserum, diluted at 1 : 1000 in 1% non-fat dried milk in 1× PBS plus 0.1% Tween20 (PBST). The membranes were developed by chemiluminescence using ECL (enhanced chemiluminescence) plus detection reagent.
Vero cells and PEDV preparation
American green monkey kidney (Vero) cells (ATCC, CCL-81) were cultured in MEM media (minimum essential media) (MEM, antibiotic-antimycotic and Na 2 CO 3 ) containing 5% FBS (fetal bovine serum) at 37 °C in a humidified 5% CO 2 . The PEDV strain CBR (GenBank accession number KJ960179) was propagated in Vero cells in MM medium containing 2 µg/mL of trypsin. The PEDV stock was harvested in Vero cells and virus titer was 10 4 –10 5 TCID50/mL.
Binding affinity analyzed by ELISA
After titration and optimal dilution of PEDV, polystyrene 96 well microtiter plate were coated with solution from the media from Vero cell as negative control and viral solution and incubated at 4 °C overnight. The plate was blocked with 5% skim milk in 1× PBS at 37 °C for 1 h and then subsequently incubated with 1 : 1000 plant 2C10 mAb diluted in 1% skim milk at 37 °C for 2 h. The plate was washed three times with PBST and incubated with HRP-conjugated anti-human IgG antiserum, Fc specific, (diluted 1 : 1000 in 1% skim milk) at 37 °C for 2 h. The plates were washed again and developed using 3, 3′, 5, 5′ t etramethy benzidine liquid substrate for 15 min at 23 °C in dark condition. The reactions were stopped with 1 N H 2 SO 4 and determined with micro-plate reader at OD 450 .
Neutralization assay
Vero cells were seeded into the 96-well plates and grown until 80% coverage of the plate. PEDV virus was diluted to TCID50 (50% tissue culture infectious dose) in MM medium and incubated with 100 µl of different dilutions of plant-made 2C10 mAb (1 : 4, 1 : 8, 1 : 16, 1 : 32, 1 : 64, 1 : 128, and 1 : 256) at 37 °C for 1 h. The Vero cells were washed with PBS one time and MM containing 10 µg/mL trypsin one time. The viral mixed solutions were transferred to the Vero cells plate and incubated at 37 °C for 1 h. Following the incubation, the solution was removed and MM plus 2% FBS was added. The Vero cells plate was incubated at 37 °C in a humidified 5% CO 2 for 5 – 7 d. Colostrum samples with and without neutralizing activity were used as positive and negative control, respectively. The assays were performed in duplicate. The cytopathic effect (CPE) were determined and compared with positive and negative control.
Acknowledgements
This work was supported by the grant from Thailand Research Fund (grant number MRG5980087 and IRN59W0001) and Thailand Agricultural Research Development Agency (Public Organization). Waranyoo Phoolcharoen was supported by the postdoctoral fellowship from the Faculty of Pharmaceutical Sciences, Chulalongkorn University (grant number Phar2559-RG07). Kaewta Rattanapisit was supported by the Ratchadaphiseksomphot Fund, Chulalongkorn University, for the postdoctoral fellowship. We thank Professor Julian Ma (St. Georgeʼs Medical School, University of London, UK) for providing the facility to produce 2C10 mAb.
Footnotes
Conflict of Interest On behalf of my co-authors, I declare that there are no financial or other conflicts of interest in the present manuscript. This work was original research, and the data presented in this article have not been published in elsewhere.
References
- 1.Pensaert M B, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q, Li G, Stasko J, Thomas J T, Stensland W R, Pillatzki A E, Gauger P C, Schwartz K J, Madson D, Yoon K J, Stevenson G W, Burrough E R, Harmon K M, Main R G, Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Li H, Liu Y, Pan Y, Deng F, Song Y, Tang X, He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis. 2012;18:1350–1353. doi: 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung H C, Lee J C, Nguyen V G, Huynh T M, Lee G E, Moon H J, Park S J, Kim H K, Park B K. New emergence pattern with variant porcine epidemic diarrhea viruses, South Korea, 2012–2015. Virus Res. 2016;226:14–19. doi: 10.1016/j.virusres.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C N, Chung W B, Chang S W, Wen C C, Liu H, Chien C H, Chiou M T. US-like strain of porcine epidemic diarrhea virus outbreaks in Taiwan, 2013–2014. J Vet Med Sci. 2014;76:1297–1299. doi: 10.1292/jvms.14-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam M T, Kubota T, Ujike M, Yahara Y, Taguchi F. Phylogenetic and antigenic characterization of newly isolated porcine epidemic diarrhea viruses in Japan. Virus Res. 2016;222:113–119. doi: 10.1016/j.virusres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheun-Arom T, Temeeyasen G, Tripipat T, Kaewprommal P, Piriyapongsa J, Sukrong S, Chongcharoen W, Tantituvanont A, Nilubol D. Full-length genome analysis of two genetically distinct variants of porcine epidemic diarrhea virus in Thailand. Infect Genet Evol. 2016;44:114–121. doi: 10.1016/j.meegid.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasey D, Cartwright S F. Virus-like particles associated with porcine epidemic diarrhoea. Res Vet Sci. 1978;25:255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocherhans R, Bridgen A, Ackermann M, Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, van Kuppeveld F J, He Q, Rottier P J, Bosch B J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016;226:117–127. doi: 10.1016/j.virusres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh J, Lee K W, Choi H W, Lee C. Immunogenicity and protective efficacy of recombinant S1 domain of the porcine epidemic diarrhea virus spike protein. Arch Virol. 2014;159:2977–2987. doi: 10.1007/s00705-014-2163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz D J, Kim C J, Shin H J. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize Porcine epidemic diarrhea virus. Virus Res. 2008;132:192–196. doi: 10.1016/j.virusres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun D, Feng L, Shi H, Chen J, Cui X, Chen H, Liu S, Tong Y, Wang Y, Tong G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet Microbiol. 2008;131:73–81. doi: 10.1016/j.vetmic.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang S H, Bae J L, Kang T J, Kim J, Chung G H, Lim C W, Laude H, Yang M S, Jang Y S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol Cells. 2002;14:295–299. [PubMed] [Google Scholar]
- 15.Cruz D J, Kim C J, Shin H J. Phage-displayed peptides having antigenic similarities with porcine epidemic diarrhea virus (PEDV) neutralizing epitopes. Virology. 2006;354:28–34. doi: 10.1016/j.virol.2006.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pyo H M, Kim I J, Kim S H, Kim H S, Cho S D, Cho I S, Hyun B H. Escherichia coli expressing single-chain Fv on the cell surface as a potential prophylactic of porcine epidemic diarrhea virus. Vaccine. 2009;27:2030–2036. doi: 10.1016/j.vaccine.2009.01.130. [DOI] [PubMed] [Google Scholar]
- 17.Tschofen M, Knopp D, Hood E, Stoger E. Plant molecular farming: much more than medicines. Annu Rev Anal Chem (Palo Alto Calif) 2016;9:271–294. doi: 10.1146/annurev-anchem-071015-041706. [DOI] [PubMed] [Google Scholar]
- 18.Topp E, Irwin R, McAllister T, Lessard M, Joensuu J J, Kolotilin I, Conrad U, Stoger E, Mor T, Warzecha H, Hall J C, McLean M D, Cox E, Devriendt B, Potter A, Depicker A, Virdi V, Holbrook L, Doshi K, Dussault M, Friendship R, Yarosh O, Yoo H S, MacDonald J, Menassa R. The case for plant-made veterinary immunotherapeutics. Biotechnol Adv. 2016;34:597–604. doi: 10.1016/j.biotechadv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Schneider J D, Castilho A, Neumann L, Altmann F, Loos A, Kannan L, Mor T S, Steinkellner H. Expression of human butyrylcholinesterase with an engineered glycosylation profile resembling the plasma-derived orthologue. Biotechnol J. 2014;9:501–510. doi: 10.1002/biot.201300229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loos A, Gach J S, Hackl T, Maresch D, Henkel T, Porodko A, Bui-Minh D, Sommeregger W, Wozniak-Knopp G, Forthal D N, Altmann F, Steinkellner H, Mach L. Glycan modulation and sulfoengineering of anti-HIV-1 monoclonal antibody PG9 in plants. Proc Natl Acad Sci U S A. 2015;112:12675–12680. doi: 10.1073/pnas.1509090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer R, Emans N, Schuster F, Hellwig S, Drossard J. Towards molecular farming in the future: using plant-cell-suspension cultures as bioreactors. Biotechnol Appl Biochem. 1999;30:109–112. [PubMed] [Google Scholar]
- 22.Chen Q, He J, Phoolcharoen W, Mason H S. Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum Vaccin. 2011;7:331–338. doi: 10.4161/hv.7.3.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phoolcharoen W, Bhoo S H, Lai H, Ma J, Arntzen C J, Chen Q, Mason H S. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol J. 2011;9:807–816. doi: 10.1111/j.1467-7652.2011.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai H, He J, Engle M, Diamond M S, Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol J. 2012;10:95–104. doi: 10.1111/j.1467-7652.2011.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai H, He J, Hurtado J, Stahnke J, Fuchs A, Mehlhop E, Gorlatov S, Loos A, Diamond M S, Chen Q. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnol J. 2014;12:1098–1107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol J. 2015;12:193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song D, Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes. 2012;44:167–175. doi: 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun R Q, Cai R J, Chen Y Q, Liang P S, Chen D K, Song C X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis. 2012;18:161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song D S, Yang J S, Oh J S, Han J H, Park B K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine. 2003;21:1833–1842. doi: 10.1016/S0264-410X(03)00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek P S, Choi H W, Lee S, Yoon I J, Lee Y J, Lee D S, Lee S, Lee C. Efficacy of an inactivated genotype 2b porcine epidemic diarrhea virus vaccine in neonatal piglets. Vet Immunol Immunopathol. 2016;174:45–49. doi: 10.1016/j.vetimm.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song D, Moon H, Kang B. Porcine epidemic diarrhea: a review of current epidemiology and available vaccines. Clin Exp Vaccine Res. 2015;4:166–176. doi: 10.7774/cevr.2015.4.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao L, Qin Z, Ge X, Yin X, Xia C, Bu R E, Fang Y, Liu J, Gao Y, Ren X. Generation of a monoclonal antibody to S1 protein of porcine epidemic diarrhea virus. Monoclon Antib Immunodiagn Immunother. 2013;32:371–374. doi: 10.1089/mab.2013.0045. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Q, Guo D, Feng L, Sun D. Expression and purification of the scFv from hybridoma cells secreting a monoclonal antibody against S PROTEIN of PEDV. Monoclon Antib Immunodiagn Immunother. 2013;32:41–46. doi: 10.1089/mab.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Nieuwstadt A P, Zetstra T. Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhea virus. Am J Vet Res. 1991;52:1044–1050. [PubMed] [Google Scholar]
- 35.Lee H M, Lee B J, Tae J H, Kweon C H, Lee Y S, Park J H. Detection of porcine epidemic diarrhea virus by immunohistochemistry with recombinant antibody produced in phages. J Vet Med Sci. 2000;62:333–337. doi: 10.1292/jvms.62.333. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Yao Y, Gao X, Wang Y, Jia X, Xiao Y, Wang T, Li X, Tian K. Development of a neutralizing monoclonal antibody against porcine epidemic diarrhea virus S1 protein. Monoclon Antib Immunodiagn Immunother. 2016;35:37–40. doi: 10.1089/mab.2015.0049. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Chen J, Wei F, Dong Y, Zhu L, Han W, Wang L, Shen Z. Prokaryotic expression of truncated S1 protein of porcine epidemic diarrhea virus and production of monoclonal antibodies to recombinant protein. Monoclon Antib Immunodiagn Immunother. 2015;34:327–333. doi: 10.1089/mab.2015.0014. [DOI] [PubMed] [Google Scholar]
- 38.Pan X, Kong N, Shan T, Zheng H, Tong W, Yang S, Li G, Zhou E, Tong G. Monoclonal antibody to N protein of porcine epidemic diarrhea virus. Monoclon Antib Immunodiagn Immunother. 2015;34:51–54. doi: 10.1089/mab.2014.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diamos A G, Rosenthal S H, Mason H S. 5′ and 3′ untranslated regions strongly enhance performance of geminiviral replicons in Nicotiana benthamiana leaves . Front Plant Sci. 2016;7:200. doi: 10.3389/fpls.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rattanapisit K, Cho M H, Bhoo S H. Lysine 206 in Arabidopsis phytochrome A is the major site for ubiquitin-dependent protein degradation. J Biochem. 2016;159:161–169. doi: 10.1093/jb/mvv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langel S N, Paim F C, Lager K M, Vlasova A N, Saif L J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): historical and current concepts. Virus Res. 2016;226:93–107. doi: 10.1016/j.virusres.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol. 2013 doi: 10.3402/jom.v5i0.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul M, Reljic R, Klein K, Drake P M, van Dolleweerd C, Pabst M, Windwarder M, Arcalis E, Stoger E, Altmann F, Cosgrove C, Bartolf A, Baden S, Ma J K. Characterization of a plant-produced recombinant human secretory IgA with broad neutralizing activity against HIV. MAbs. 2014;6:1585–1597. doi: 10.4161/mabs.36336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiatt A, Ma J K. Monoclonal antibody engineering in plants. FEBS Lett. 1992;307:71–75. doi: 10.1016/0014-5793(92)80904-u. [DOI] [PubMed] [Google Scholar]
- 45.Ma J K, Lehner T, Stabila P, Fux C I, Hiatt A. Assembly of monoclonal antibodies with IgG1 and IgA heavy chain domains in transgenic tobacco plants. Eur J Immunol. 1994;24:131–138. doi: 10.1002/eji.1830240120. [DOI] [PubMed] [Google Scholar]
- 46.Both L, van Dolleweerd C, Wright E, Banyard A C, Bulmer-Thomas B, Selden D, Altmann F, Fooks A R, Ma J K. Production, characterization, and antigen specificity of recombinant 62-71-3, a candidate monoclonal antibody for rabies prophylaxis in humans. FASEB J. 2016;27:2055–2065. doi: 10.1096/fj.12-219964. [DOI] [PMC free article] [PubMed] [Google Scholar]


