Abstract
Swine influenza virus (SIV) and Mycoplasma hyopneumoniae (Mhp) are widespread in farms and are major pathogens involved in the porcine respiratory disease complex (PRDC). The aim of this experiment was to compare the pathogenicity of European avian-like swine H1N1 and European human-like reassortant swine H1N2 viruses in naïve pigs and in pigs previously infected with Mhp. Six groups of SPF pigs were inoculated intra-tracheally with either Mhp, or H1N1, or H1N2 or Mhp + H1N1 or Mhp + H1N2, both pathogens being inoculated at 21 days intervals in these two last groups. A mock-infected group was included. Although both SIV strains induced clinical signs when singly inoculated, results indicated that the H1N2 SIV was more pathogenic than the H1N1 virus, with an earlier shedding and a greater spread in lungs. Initial infection with Mhp before SIV inoculation increased flu clinical signs and pathogenesis (hyperthermia, loss of appetite, pneumonia lesions) due to the H1N1 virus but did not modify significantly outcomes of H1N2 infection. Thus, Mhp and SIV H1N1 appeared to act synergistically, whereas Mhp and SIV H1N2 would compete, as H1N2 infection led to the elimination of Mhp in lung diaphragmatic lobes. In conclusion, SIV would be a risk factor for the severity of respiratory disorders when associated with Mhp, depending on the viral subtype involved. This experimental model of coinfection with Mhp and avian-like swine H1N1 is a relevant tool for studying the pathogenesis of SIV-associated PRDC and testing intervention strategies for the control of the disease.
Keywords: Swine influenza virus, Mycoplasma hyopneumoniae, Porcine respiratory disease complex, Pigs, Co-infection
1. Introduction
Swine influenza is an acute respiratory tract infection in pigs, characterized by high fever, depression, loss of appetite, tachypnoea, dyspnea and coughing. At the microscopic level, the infection induces interstitial pneumonia and bronchiolitis. Pathogens causing swine flu are type A influenza viruses. Three swine influenza virus (SIV) subtypes, i.e. H1N1, H1N2 and H3N2, are circulating among pigs worldwide, whereas lineages may vary within each subtype depending on the region, i.e. North America, Europe and Asia. The disease is highly contagious. The morbidity can be high (near 100%) but the mortality rate is usually low (less than 1%) (Olsen et al., 2006). A flu outbreak has an important economic impact particularly because of the medications costs and the growth rate decrease (Bardini et al., 2011, Brons et al., 2011). These financial losses vary depending on the severity of the disease, which is influenced by many factors: the virus strain, the age and immune status of the infected pig, the presence of concurrent infections, climatic conditions, housing and infection pressure.
SIVs have been also recognized to be viral pathogens involved in the porcine respiratory disease complex (PRDC). PRDC is characterized by decreased rate of growth, decreased feed efficiency, anorexia, fever, cough and dyspnea. It is a multifactorial disease of pigs caused by sequential or concurrent infections with several viral or bacterial respiratory pathogens. Together with SIVs, some pathogens involved in PRDC have been identified to be Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Porcine Circovirus type-2 (PCV-2), Porcine Respiratory Coronavirus (PRCV), Mycoplasma hyopneumoniae (Mhp), Pasteurella multocida, Streptococcus suis, Haemophilus parasuis and Actinobacillus pleuropneumoniae (Choi et al., 2003, Sorensen et al., 2006, Thacker, 2001). Several studies based on experimental dual infections of pigs have been conducted to understand interactions between SIVs and PRRSV, PCV-2, Mhp, PRCV or Bordetella bronchiseptica, pathogens that are frequently detected in herds (Lanza et al., 1992, Loving et al., 2010, Thacker et al., 2001, Van Reeth et al., 1996, Van Reeth et al., 2001, Van Reeth and Pensaert, 1994, Wei et al., 2010, Yazawa et al., 2004). Among them, Mhp is one of the pathogens that is most commonly isolated from pigs with clinical signs of PRDC. Alone, Mhp induces chronic pneumonia with dry, non productive cough beginning 7–14 days after infection (Thacker, 2006). The hallmark lesions associated with Mhp are hyperplasia of the bronchus associated lymphoid tissue (BALT) and bronchointerstitial pneumonia. Experimental co-infections with Mhp and SIVs from the “classical swine H1N1” lineage mostly circulating in America suggested that the dual infection results in increased overall pathogenicity as compared to outcomes of individual infections (Thacker et al., 2001, Yazawa et al., 2004).
Despite the extensive use of M. hyopneumoniae vaccination in France, Mhp is widespread in French farms. An epidemiological survey in French farms showed that Mhp was detected in 1/3 of herds at 4 weeks of age (Fablet et al., 2012). Also, SIVs of the European “avian-like swine H1N1” and “human-like reassortant swine H1N2” lineages are currently circulating in nearly half of French herds (Kuntz-Simon and Madec, 2009, Kyriakis et al., 2011) and epidemiological investigations suggested that the presence of Mhp may influence the outcomes of subsequent infections with SIVs (Fablet et al., 2012). In order to go further in understanding interactions between these two pathogens, we intended to compare outcomes of infection with European H1N1 and H1N2 SIVs, in naïve pigs or in pigs previously infected with Mhp.
2. Materials and methods
2.1. Viruses and Mycoplasma strain
The SIV strains A/Sw/Cotes d’Armor/0231/06 (H1N1) and A/Sw/Cotes d’Armor/0113/06 (H1N2) were isolated from nasal swabs taken from pigs from outbreaks of acute respiratory disease in French herds. They were isolated onto Madin Darby Canine Kidney (MDCK) cells and further propagated in the allantoic cavity of 9-day-old embryonated chicken eggs at 36 °C for 3 days for inoculum production, following standard procedure (OIE, 2008). Allantoic fluids were tested for haemagglutinating activity with 0.5% chicken erythrocytes. Virus titer was determined by inoculating 5 embryonated chicken eggs with 150 μl of 10-fold serial dilutions of the virus stock. After 9 days of incubation at 36 °C, the embryonic lethal dose (ELD50/ml) was calculated by the method of Reed and Muench.
M. hyopneumoniae (Mhp, strain 116) was isolated from an outbreak of enzootic pneumonia in France and cultivated in Friis broth medium (FBM) at 37 °C (Marois et al., 2007). Three 10-fold serial dilutions of the Mhp stock were prepared in FBM and incubated at 37 °C. After 5–10 days, color changes of medium were observed and the Mhp stock titer was calculated and expressed as color-changing units per milliliter (CCU/ml).
2.2. Animals and experimental design
Forty specific pathogen-free 6-week-old pigs were randomly allocated into study groups. The animals were obtained from the experimental pig herd of the French Agency for Food, Environmental and Occupational Health and Safety (Anses) at Ploufragan. These animals were known to be free from SIV and Mhp at the beginning of the study. Experiments were performed in accordance with the animal welfare experimentation recommendation granted by the Direction des Services Vétérinaires des Côtes d’Armor (Anses registration number B-22-745-1), under the responsibility of G. Simon (authorization number 22-26).
Twenty pigs were inoculated intra-tracheally with Mhp 116 (5 × 108 CCU in a total of 5 ml), while twenty others were inoculated intra-tracheally with 5 ml of FBM. These operations were repeated twice during a 24 h period. Three weeks later, i.e. at day 21, 5 Mhp-infected animals and 5 mock-infected animals were inoculated intra-tracheally with 5 × 105 ELD50 in a total of 5 ml of SIV H1N1 (MH1N1 and H1N1 groups, respectively). 5 other Mhp-infected animals and 5 other mock-infected animals were inoculated intra-tracheally with 5 × 105 ELD50 in a total of 5 ml of SIV H1N2 (MH1N2 and H1N2 groups, respectively). Simultaneously, the last 10 Mhp-infected and the last 10 mock-infected animals were inoculated intra-tracheally with 5 ml of allantoic fluid (M and C groups, respectively) (Table 1 ).
Table 1.
Experimental design.
| Groups | No. of pigs | Inoculations |
Day of necropsy | |
|---|---|---|---|---|
| Day 0 + Day 1 | Day 21 | |||
| C | 10 | Friis broth medium | Allantoic fluid | Day 31 |
| M | 10 | Mycoplasma hyopneumoniae | Allantoic fluid | Day 30 |
| H1N1 | 5 | Friis broth medium | H1N1 | Day 28 |
| MH1N1 | 5 | Mycoplasma hyopneumoniae | H1N1 | Day 29 |
| H1N2 | 5 | Friis broth medium | H1N2 | Day 28 |
| MH1N2 | 5 | Mycoplasma hyopneumoniae | H1N2 | Day 29 |
C, control group; M, Mhp-inoculated group; H1N1, SIV H1N1-inoculated group; MH1N1, Mhp and SIV H1N1-inoculated group; H1N2, SIV H1N2-inoculated group; MH1N2, Mhp and SIV H1N2-inoculated group. Pigs were 6-weeks-old at Day 0. Inoculations were made intra-tracheally.
2.3. Clinical observation and sampling
Clinical signs including cough (number of coughs for 15 min), respiratory rate (number of breathing of 2 pigs per group for 1 min) and rectal temperature were evaluated daily, throughout the study. Pigs with rectal temperatures >40 °C were considered to be febrile. Pigs were weighted weekly for four weeks. Nasal swabs were taken at days 23, 25 and 28, i.e. 2, 4 and 7 days post-infection (DPI) with SIV. After collection, swabs were suspended in 2 mL of Eagle's minimum essential medium (EMEM, LONZA, Levallois-Perret, France) containing penicillin and streptomycin (Sigma, Saint-Quentin Fallavier, France), vigorously vortexed and supernatants were aliquoted and stored at −70 °C. Blood samples were collected weekly. Sera were separated by centrifugation and stored at −20 °C until use.
2.4. Necropsy and macroscopic examination of lung
Animals were euthanised by intravenous booster of sodium pentobarbital at the end of experiment. Pigs from H1N1 and H1N2 groups were necropsied at day 28, i.e. 7 DPI with SIV. Pigs from MH1N1 and MH1N2 groups were similarly necropsied at 29 DPI, pigs from M group at 30 DPI and pigs from C group at 31 DPI. Lungs were removed in toto and macroscopic lesions (purple-red and firm lesions) were estimated visually for each lobe. A score between 0 and 4 was assigned to each of the seven lobes (0 = no lesions; 4 = the entire lobe is affected). Thus, the maximum total score possible for each lung was 28 (Madec and Kobisch, 1982).
The right lung was used for bronchoalveolar lavage (BAL). The lung was flushed with 3 × 20 ml of phosphate buffer saline (PBS). Recovered BAL fluids (BALFs) were stored at −70 °C until use. A sample of each lobe on the left-hand part of the lung (left caudal, left cardiac and left diaphragmatic) was collected for the detection of SIV and Mhp. Samples collected from the diaphragmatic lobe were fixed in 10% neutral buffered formalin for histopathological examination.
2.5. Histopathologic evaluation
For histopathological examination, formalin-fixed tissue samples were embedded in paraffin wax, sectioned and stained with haematoxylin, eosin and safran. Sections were evaluated by light microscopy for histopathological changes. Lesion severity was scored as following: 0, no lesion; 1, mild; 2, moderate; 3, marked; 4, very marked.
2.6. SIV detection
Nasal swab supernatants, BALFs and lung samples were analysed by matrix (M) gene real-time RT-PCR. Briefly, RNA was extracted from 200 μl of nasal swab specimen or BALF or from 30 mg of organ with the NucleoSpin RNA II kit or the NucleoSpin 8 RNA kit (Macherey-Nagel, Hoerdt, France). 2.5 μl of RNA template was tested using a one step RT-PCR kit (Qiagen, Courtaboeuf, France) in a total volume of 25 μl. Primers and probe were previously described (Spackman et al., 2002). The real-time RT-PCR was run in a Chromo4 Real-Time Detector (Bio-Rad, Marnes-la-Coquette, France) and the thermal profile used was 50 °C for 30 min followed by 95 °C for 15 min and 40 cycles of 95 °C for 10 s and 60 °C for 20 s.
2.7. Virus titration on MDCK cells
Infectious SIV particles secreted in nasal swab supernatants were titrated in MDCK cells. Cells were seeded 24 h before titration in 96-well plates at a density of 3 × 104 cells/well in EMEM supplemented with 5% bovine calf serum (BCS), 1% tricine (Sigma, Saint-Quentin Fallavier, France), penicillin and streptomycin. Tenfold serial dilutions of sample, starting at 1:10, were prepared in cell culture infection medium, i.e. EMEM without BCS, supplemented with 2 μg/ml of trypsin-TPCK (Worthington Biochemical Corporation, Lakewood, NJ, USA), 1% tricine, penicillin and streptomycin. Cells were washed twice with EMEM and then inoculated with the dilution (8 replicas per dilution). After 3 and 4 days of incubation at 37 °C, the presence of cytopathogen effect (CPE) was observed and SIV infectivity titers were calculated by the method of Reed and Muench.
2.8. Mhp DNA detection
DNA was extracted from 25 mg of lung samples (apical and diaphragmatic lobes) with DNeasy Blood and Tissue kit (Qiagen, Courtaboeuf, France). Mhp was detected and quantified by PCR, as previously described (Marois et al., 2010).
2.9. Serology
Sera were tested for SIV antibodies by haemagglutination inhibition (HI) assays. Sera were treated with the receptor-destroying enzyme from Vibrio cholera (Sigma, Saint-Quentin Fallavier, France) to remove nonspecific inhibitors of the agglutination and with a 50% chicken red blood cell (RBC) suspension to remove nonspecific agglutinins. Samples were then serially diluted with PBS in 96-well plates and four haemagglutinating units of virus per 25 μl were added to each well. The virus antigens used in the HI assays were the challenge viruses, A/Sw/Cotes d’Armor/0231/06 (H1N1) and A/Sw/Cotes d’Armor/0113/06 (H1N2). A 0.5% chicken RBC suspension was added and HI titers were read after 30 to 45 min at room temperature. HI titers ≥ 20 were recorded as positive.
2.10. Statistics
Data were compared by Kruskall–Wallis test. Statistical analyses were performed using Systat 9 software. Differences were considered significant when P ≤ 0.05.
3. Results
3.1. Clinical disease
All groups infected with Mhp, i.e. M, MH1N1 and MH1N2 groups, developed coughing from 14 days after Mhp infection (Table 2 ). By contrast, coughing was rare within pigs that were not infected with Mhp during that period. SIV H1N1 and H1N2 infections did not induce coughing by themselves and had no significant impact on the severity of coughing score measured in groups previously infected with Mhp during the first 5 days post-SIV infection, i.e. between the 21st and the 25th day following Mhp inoculation. However, between the 28th and the 29th day after Mhp infection, so 1 week after SIV inoculation, coughing was significantly different in the MH1N2 group as compared to that observed in M and MH1N1 groups. Indeed, 5/5 pigs were coughing within MH1N2 whereas only 7/10 and 2/5 pigs in M and MH1N1 groups, respectively. Also, the frequency of coughing was higher in the MH1N2 group.
Table 2.
Coughing after pathogen inoculations, alone or in co-infection.
| Groups | No. of pigs | Days after Mhp inoculation (SIV inoculation at day 21) |
||||
|---|---|---|---|---|---|---|
| 0–4 | 7–11 | 14–18 | 21–25 | 28–29 | ||
| C | 10 | 1 (0.0 ± 0.1) | 0 | 0 | 0 | 0 |
| M | 10 | 0 | 1 (0.0 ± 0.1) | 6 (0.5 ± 1.0) | 10 (0.8 ± 0.9) | 7 (1.1 ± 1.0) |
| H1N1 | 5 | 0 | 1 (0.0 ± 0.2) | 0 | 1 (0.1 ± 0.3) | 0 |
| MH1N1 | 5 | 0 | 1 (0.0 ± 0.2) | 3 (0.6 ± 0.8) | 5 (1.2 ± 1.1)* | 2 (0.5 ± 0.9) |
| H1N2 | 5 | 0 | 0 | 0 | 2 (0.1 ± 0.3) | 0 |
| MH1N2 | 5 | 0 | 0 | 1 (0.3 ± 0.5) | 5 (0.9 ± 1.4)* | 5 (1.9 ± 1.4)** |
Number of pigs with coughing and, in brackets, mean of coughing per pig and per 15 min ± standard deviation. The mean is calculated from daily counts made during the five days of the week except for the last week where the average was calculated over two days (28 and 29 DPI). SIV infection was performed 21 days after Mhp inoculation. C, control group; M, Mhp-inoculated group; H1N1, SIV H1N1-inoculated group; MH1N1, Mhp and SIV H1N1-inoculated group; H1N2, SIV H1N2-inoculated group; MH1N2, Mhp and SIV H1N2-inoculated group.
MH1N1 and MH1N2 are significantly different from H1N1 and H1N2 respectively (P ≤ 0.05).
MH1N2 group is significantly different from M, and MH1N1 groups (P ≤ 0.05).
All pigs had normal rectal temperature during the first three weeks of the experimental assay. Pyrexia (>40 °C) was observed in the four groups infected with SIV at 1 DPI SIV. At 2 DPI SIV, mean rectal temperature in MH1N1 group was still significantly different from that of the C group (Fig. 1 ). A second hyperthermia peak appeared at 4 and 5 DPI in some pigs from co-infected groups, leading MH1N1 and MH1N2 mean rectal temperatures to be again significantly different from that of the C group at 4 DPI.
Fig. 1.
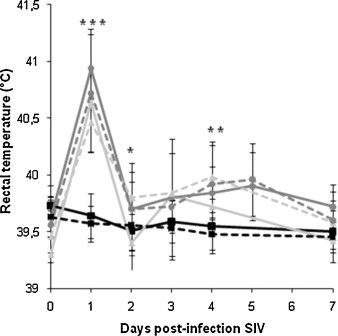
Mean rectal temperature (°C) of pigs after SIV inoculation. Mean ± standard deviation of rectal temperature of C group ( ), M group (
), M group ( ), H1N1 group (
), H1N1 group ( ), MH1N1 group (
), MH1N1 group ( ), H1N2 group (
), H1N2 group ( ) and MH1N2 group (
) and MH1N2 group ( ). *** indicates that all SIV-infected groups are significantly different from C group (P ≤ 0.05). ** indicates that MH1N2 and MH1N1 are significantly different from C group (P ≤ 0.05). * indicates that only MH1N1 is significantly different from C group (P ≤ 0.05).
). *** indicates that all SIV-infected groups are significantly different from C group (P ≤ 0.05). ** indicates that MH1N2 and MH1N1 are significantly different from C group (P ≤ 0.05). * indicates that only MH1N1 is significantly different from C group (P ≤ 0.05).
Other clinical signs were observed after SIV inoculation. Pigs of the H1N1 group showed some lethargy and inappetence, but these symptoms were not severe and recovery was evident at 2 DPI except for 1/5 animal. In animals that were previously infected with Mhp (MH1N1 group), these symptoms were amplified, especially inappetence. One pig over 5, that maintained a normal rectal temperature throughout the study, was the most affected. Its decline was characterized by vomiting, accelerated abdominal breathing and severe anorexia. Over the week post-infection with SIV, pigs from the MH1N1 group ate food in an average of 0.971 kg/day/pig, whereas pigs in the H1N1 group ate 1.297 kg/day/pig. By comparison, pigs in C and M groups ate 1.782 kg/day/pig and 1.702 kg/day/pig, respectively. It has to be noted that the food consumption decrease following SIV H1N1 infection was particularly marked from 0 to 4 DPI. As a consequence, the mean weight gain (MWG) was significantly reduced in the H1N1 group, as compared to the C group, when calculated for the first 4 days period post-SIV infection (Fig. 2 ). Whereas Mhp infection alone did not affect normal weight gain, pre-infection with Mhp led H1N1-inoculated pigs to a very strong weight gain reduction, resulting in a negative MWG, i.e. a weight loss over the first 4 days post-SIV H1N1 infection.
Fig. 2.
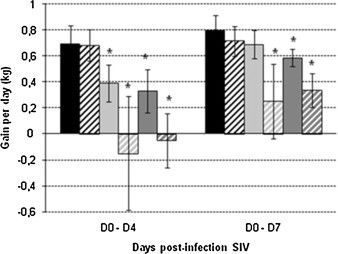
Mean daily weight gain (kg) of pigs after SIV inoculation, between 0 and 4 days post-infection and between 0 and 7 days post-infection. Mean daily weight gain of C group ( ), M group (
), M group ( ), H1N1 group (
), H1N1 group ( ), MH1N1 group (
), MH1N1 group ( ), H1N2 group (
), H1N2 group ( ) and MH1N2 group (
) and MH1N2 group ( ). * indicates that the group is significantly different from the C group (P ≤ 0.05).
). * indicates that the group is significantly different from the C group (P ≤ 0.05).
Pigs infected with the SIV H1N2 strain also presented clinical outcomes as lethargy, frail and anemic look, vomiting, increased respiratory rates and weight gain reduction. All these symptoms induced by SIV H1N2 were more pronounced than those observed in pigs infected with SIV H1N1 alone. Thus, the MWG reduction was still significant when calculated from 0 to 7 days post-H1N2 inoculation, whereas it was not found significant after H1N1 infection over that period of time (Fig. 2). Previous infection with Mhp increased the severity of clinical signs induced by SIV H1N2, but to a lesser extent than it had on pigs infected with H1N1. Over the 7 days post-infection with SIV, pigs in MH1N2 group ate 1.051 kg of food in average against 1.302 kg in H1N2 group. However, whereas the difference in food consumption between these two groups was equivalent to that observed between MH1N1 and H1N1 groups, the weight loss was less important in MH1N2 group than in MH1N1 group. Previous infection with Mhp also affected the respiratory rate since pigs in MH1N2 group had flank movements more pronounced than in H1N2 group. Respiratory rate in MH1N2 group reached 105–110 breaths per minute while in H1N2 group the maximum rate was 77–80 breaths per minute and only 40–44 in the control group.
3.2. Macroscopic and microscopic lesions
As shown in Fig. 3 , mock-infected control animals had no macroscopic lung lesions. Pneumonia induced by Mhp and/or SIV infection was characterized by plum colored and consolidated areas on lobes. Each group infected with Mhp or/and SIV had a macroscopic lesion score significantly higher than the control group. No significant difference was found between the scores of H1N1 and H1N2 groups. However, it has to be noticed that the lung lesions observed in animals infected with H1N1 alone were restricted to upper lobes, whereas diaphragmatic lobes were also affected in lungs of H1N2-infected animals. Coinfection with Mhp caused a significant increase in the mean lesion score calculated for the MH1N1 group, as compared to the H1N1 group, but not for the MH1N2 group, as compared to the H1N2 group. In MH1N1 group, diaphragmatic lobes became affected, whereas in the MH1N2 group the lesion distribution was similar as that described in the H1N2 group.
Fig. 3.
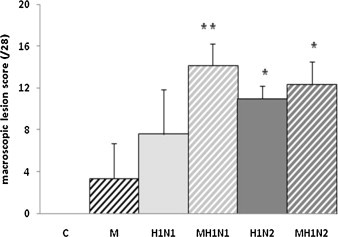
Average score of macroscopic lung lesions. Each lobe was scored visually between 0 and 4 (0 = no lesions; 4 = the entire lobe is affected). The scores for each lobe were summed for an overall score of the entire lung (score/28) of each animal, and then the average score was calculated for the group. * indicates that the group is significantly different from M group (P ≤ 0.05). ** indicates that the group is significantly different from M group and from H1N1 group (P ≤ 0.05).
Microscopic lesions were also observed and scored (Fig. 4 and Table 3 ). The lungs of pigs in the control group showed mild lesions but these lesions were not specific of Mhp nor SIV infection (Fig. 4(a)). In Mhp-infected group, 4/10 pigs developed a broncho-interstitial pneumonia with a hyperplasia of the bronchiolar-associated lymphoid tissue, that is due to Mhp infection (Fig. 4(b)). Groups infected with SIVs developed subacute bronchiolitis, sometimes acute with cell necrosis. These lesions were typical from SIV infections but the scores varied depending on the virus strains: H1N1-infected pigs showed moderated to marked lesions (Fig. 4(c)) whereas pigs infected with H1N2 showed mild to moderated lesions (Fig. 4(e)). In the H1N1 group, the bronchiolitis was accompanied by interstitial pneumonia, with hypertrophy of peribronchiolar smooth muscle more important than in the H1N2 group.
Fig. 4.
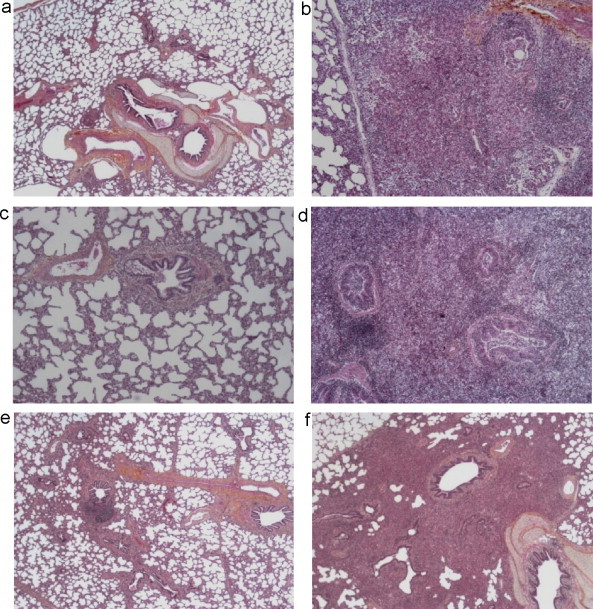
Hematoxylin eosin and safran stained sections of lungs. (a) A mock-challenged pig (C group), (b) a pig infected with Mhp (M group), (c) a pig infected with H1N1 (H1N1 group), (d) a pig co-infected with Mhp and H1N1 (MH1N1 group), (e) a pig infected with H1N2 (H1N2 group) and (f) a pig co-infected with Mhp and H1N2 (MH1N2 group). Magnification: ×25 for (a), (e) and (f), ×50 for (b), (c) and (d).
Table 3.
Mean histopathology lung scores.
| Groups | Hyperplasia of bronchial epithelium (score/4) | Bronchiolar-associated lymphoid tissue hyperplasia (score/4) | Interstitial pneumonia (score/4) | Bronchiolitis (score/4) | Cellular exudates in alveoli and bronchiolar lumen (score/4) | Total score/20 |
|---|---|---|---|---|---|---|
| C | 2 ± 0.8 | 0.75 ± 1 | 1.5 ± 0.8 | 0.5 ± 0.6 | 0.5 ± 0.6 | 5.3 ± 1.6 |
| M | 2.9 ± 0.7 | 0.8 ± 1.1 | 1.6 ± 0.6 | 0.9 ± 1.2 | 0.7 ± 1.5 | 6.9 ± 3.5 |
| H1N1 | 2.6 ± 1.1 | 1 ± 0.7 | 2.4 ± 0.8 | 2.2 ± 0.4* | 0.6 ± 0.5 | 8.8 ± 2.1* |
| MH1N1 | 2.6 ± 0.5 | 1.2 ± 1.3 | 1.8 ± 0.6 | 2.4 ± 1.3 | 2.4 ± 2.2 | 10.4 ± 3* |
| H1N2 | 2.8 ± 0.4 | 1 ± 1 | 1.9 ± 0.8 | 1.2 ± 1.6 | 0.2 ± 0.4 | 7.1 ± 2 |
| MH1N2 | 2.4 ± 0.9 | 2.2 ± 1.6 | 1.7 ± 0.2 | 1.4 ± 1.5 | 1 ± 1.2 | 8.7 ± 3.3* |
Microscopic lesion severity was scored as follows: 0, no lesions; 1, mild; 2, moderate; 3, marked; 4, very marked.
Group is significantly different from C group (P ≤ 0.05).
The lungs of MH1N1 pigs showed an exacerbation of bronchial pneumonia and superinfection with cellular exudates in the alveoli (Fig. 4(d)), more marked than in the lungs of MH1N2 pigs (Fig. 4(f)). However, we noted a hyperplasia of the bronchiolar-associated lymphoid tissue more important in the MH1N2 group than in the other groups. In accordance with macroscopic lung lesions, microscopic histopathology showed a stronger lung damage for MH1N1 (total score: 10.4/20) than for the other groups.
3.3. SIV isolation and virus RNA detection
SIV isolation and titration performed on nasal swabs collected at 2, 4 and 7 DPI SIV showed that the virus was shed from all SIV-infected groups from 2 DPI, but frequencies of excreting animals and amounts of excreted virus varied depending on the inoculated pathogen(s) (Table 4 ). Thus, 5/5 animals in the H1N2 group shed virus at 2 DPI whereas only 3/5 animals in the H1N1 group. The peak of viral shedding in nasal swabs was the fourth DPI for all groups. Virus isolation attempts and SIV RNA detection were successful for the majority of animals infected with H1N1 at 7 DPI whereas only 1/5 animals was positive in H1N2 and MH1N2 groups. By contrast to nasal swab supernatants, lung tissue samples and BALFs were not positive for virus isolation, but SIV matrix (M) gene was detected by RT-PCR in diaphragmatic lobe at 7 DPI in 4/5 animals coinfected with Mhp and H1N1, but not in animals from the H1N1 group. At that time, RNA was also detected in 3/5 and 2/5 pigs in the H1N2 and the MH1N2 groups, respectively.
Table 4.
SIV isolation and virus RNA detection.
| Groups | Pig | Virus isolationa |
Virus RNA detectionb |
BALF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasal swabs |
Nasal swabs |
Lung tissue |
|||||||||
| 2 DPI | 4 DPI | 7 DPI | 2 DPI | 4 DPI | 7 DPI | Apical lobe | Cardiac lobe | Diaphragmatic lobe | |||
| H1N1 | 1 | + (–) | + (5.1) | + (nd) | + | + | + | nt | nt | − | − |
| 2 | − (nd) | + (2.8) | + (–) | − | + | + | nt | nt | − | + | |
| 3 | − (nd) | + (3.4) | + (–) | − | + | + | nt | nt | − | + | |
| 4 | + (2.8) | + (4.7) | − (nd) | + | + | + | nt | nt | − | + | |
| 5 | + (nd) | + (4.6) | − (nd) | + | + | + | nt | nt | − | + | |
| MH1N1 | 6 | + (2.2) | + (4.5) | − (nd) | + | + | + | + | nt | + | + |
| 7 | + (3.3) | + (3.7) | − (nd) | + | + | + | + | nt | + | + | |
| 8 | + (3.4) | + (3.7) | − (nd) | + | + | − | + | nt | + | − | |
| 9 | − (nd) | + (4.6) | + (–) | − | + | + | + | nt | − | − | |
| 10 | + (–) | + (5.6) | − (nd) | + | + | + | + | nt | + | + | |
| H1N2 | 11 | + (nd) | + (3.0) | − (nd) | + | + | − | − | + | + | + |
| 12 | + (2.0) | + (4.5) | − (nd) | + | + | − | + | + | − | + | |
| 13 | + (nd) | + (3.8) | − (nd) | + | + | − | − | + | − | + | |
| 14 | + (5.0) | + (5.0) | − (nd) | + | + | + | + | + | + | − | |
| 15 | + (3.4) | + (2.8) | + (nd) | + | + | − | − | + | + | + | |
| MH1N2 | 16 | + (–) | + (5.0) | − (nd) | − | + | − | − | − | + | − |
| 17 | − (nd) | + (4.7) | − (nd) | − | + | − | + | + | − | − | |
| 18 | + (–) | + (4.8) | − (nd) | + | + | − | + | + | − | + | |
| 19 | + (2.1) | + (5.0) | − (nd) | + | + | − | − | + | + | − | |
| 20 | + (2.5) | + (5.7) | − (nd) | + | + | + | − | − | − | + | |
Results of the virus isolation in nasal swabs of pigs inoculated with SIV strains collected at 2, 4 and 7 days post-infection (DPI). + and −, positive or negative virus isolation. In brackets, virus titers expressed in TCID50/ml; –, virus titer determined to be <2 log 10 TCID50/ml; nd, not determined.
Results of the real-time RT-PCR targeting the matrix protein in nasal swabs, lung tissue and BALF from animals infected with SIV strain. + and −, presence or absence of SIV genome. nt, no tested.
3.4. Mhp detection
The Mhp genome was quantified by quantitative real-time PCR in apical and diaphragmatic lung lobes collected at necropsy in M, MH1N1 and MH1N2 groups (Fig. 5 ). Thus, Mhp DNA was detected in all Mhp-inoculated animals. About 104 UFC/25 mg of organ was quantified in apical lobes in every 3 tested groups. Concerning the diaphragmatic lobes, the quantity of DNA was significantly less important in the MH1N2 group than in the M or MH1N1 groups. There was no difference between M and MH1N1 groups.
Fig. 5.
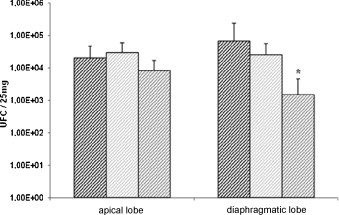
Quantification of Mhp in lungs of infected pigs using PCR at necropsy (see Table 1).  M group;
M group;  MH1N1 group and
MH1N1 group and  MH1N2 group. * indicates that the group is significantly different from M group (P ≤ 0.05).
MH1N2 group. * indicates that the group is significantly different from M group (P ≤ 0.05).
3.5. Serology
In order to study the seroconversion subsequent to SIV infection, HI tests were performed on sera collected at 7 DPI. In the H1N1 group, only 2/5 animals had developed antibodies at 7 DPI. In the other groups 5/5 animals presented antibodies but the mean HI titers differed depending on the group (Table 5 ). HI antibody titer was significantly higher when animals were infected with H1N2 than with H1N1. Co-infection with Mhp impacted the serum HI response to SIV infection: HI titers were about 3 times higher for MH1N1 and MH1N2 groups than for H1N1 and H1N2 groups, respectively. Moreover, HI antibody titer was significantly greater for MH1N2 than for MH1N1.
Table 5.
Serum haemagglutinin-inhibition titers towards the homologous challenged SIV strain at 7 days post-infection SIV.
| Groups | Mean HI titers (min–max) |
|---|---|
| H1N1 | 30 (10–40)* |
| MH1N1 | 96 (20–320) |
| H1N2 | 176 (80–320) |
| MH1N2 | 480 (160–640)** |
Group is significantly different from H1N2 and MH1N2 groups (P ≤ 0.05).
Group is significantly different from all other groups (P ≤ 0.05). The results of non SIV-infected groups are not included.
4. Discussion
The purpose of this study was to compare the pathogenesis of European avian-like swine H1N1 virus and that of European human-like reassortant swine H1N2 virus and to investigate their potential interactions with another respiratory pathogen, i.e. Mycoplasma hyopneumoniae. We have shown that both SIV subtypes were able to induce typical signs of swine influenza when inoculated intra-tracheally to naïve SPF pigs, as previously proposed by others (Haesebrouck et al., 1985, Maes et al., 1984). The experimental diseases induced by the selected H1N1 and H1N2 strains globally mimicked respiratory outbreaks observed in the herds of origin, with fever, coughing, dyspnea and loss of appetite. However, a difference in the severity of clinical and pathological effects was observed depending on the strain subtype in experimental conditions, i.e. without any involvement of external factors. Thus, the H1N2 strain appeared slightly more pathogenic than the H1N1 strain: the daily weight gain per day between 0 and 7 days after SIV inoculation was lower and macroscopic pulmonary lesions were more extended, affecting the diaphragmatic lobes. H1N2 has been detected and isolated at 2 DPI SIV in nasal swabs from 5/5 inoculated animals whereas H1N1 has been detected in only 3/5 pigs at that time. This suggested that H1N2 SIV replicated faster and stronger than H1N1 SIV, leading to earlier shedding. However, at 7 DPI, SIV was still detected in 5/5 H1N1-infected pigs but in only 1/5 H1N2-infected pigs. This would imply a faster H1N2 virus elimination, possibly related to the induction of an earlier and/or more efficient immune response after H1N2 infection as compared to H1N1 infection, as revealed by higher HI titers in H1N2 group than in H1N1 group at 7 DPI. This difference in HI titers after European H1N1 versus H1N2 SIVs confirmed observations made at 2 weeks post inoculation SIV in a previous study (Van Reeth et al., 2006).
In 2001, Thacker et al. showed that the intra-tracheal administration of Mhp to conventional pigs 21 days before the inoculation by nebulization of a classical swine H1N1 virus led to an increase in clinical signs severity and pneumonia lesions at the macroscopic level, but no incidence was reported at the microscopic level (Thacker et al., 2001). In 2004, Yazawa et al. inoculated intranasally Mhp-infected caesarian derived and colostrums deprived pigs with classical swine H1N1 and also observed an increase in lung lesions, while the SIV was slightly pathogenic when it was inoculated singly (Yazawa et al., 2004). However, these authors showed that the expression of clinical signs may depend on the time of SIV inoculation following Mhp infection, as they were less pronounced when SIV was inoculated 7 days after Mhp, as compared to those observed when both infections were performed 21 days apart. In the present study, we confirmed that intra-tracheal inoculation of an European avian-like swine H1N1 increased clinical signs and lung lesions severity as compared to those observed after H1N1 SIV inoculation in naïve pigs when inoculated 21 days after Mhp, a time where Mhp infection is well established (Marois et al., 2010). Interestingly, the most significant sign was the important weight loss over the 4 days following the H1N1 SIV inoculation, which was not determined in previous studies. There was a large variation of clinical disease between pigs in MH1N1 group. However, this individual variation was also found in other experimental studies of dual infection, as in PRRSV-SIV infection (Van Reeth et al., 1996, Van Reeth et al., 2001) but factors responsible of the variation are still unknown. Lungs were also more affected in MH1N1 group as compared to H1N1 group; lesion of pneumonia were much more extended and reached the diaphragmatic lobes. This can be correlated with the fact that H1N1 SIV was detected in diaphragmatic lobe in coinfected animals whereas it was not in singly inoculated pigs. Thus, the primary infection with Mhp seemed to impact the spread and/or persistence of the European avian-like H1N1 virus in lungs, whereas the shedding in nasal secretions was not enhanced.
The clinical effects of H1N2 infection were also enhanced by previous Mhp inoculation, as compared to H1N2 outcomes in naïve pigs, but in a lower proportion than the difference observed in case of H1N1 infections, with or without Mhp. The respiratory symptoms were increased and the animal growth was slowed. The hyperplasia of bronchiolar-associated lymphoid tissue was more important and the serum HI response to H1N2 SIV infection was higher in the MH1N2 group than in H1N2 group. That could be the sign of an immune response more advanced or higher. The present data showed a difference in Mhp genome amount in diaphragmatic lung lobes of dual infected pigs, as compared to pigs only infected with Mhp. Thus, Mhp infection itself would be also influenced by the H1N2 infection.
Altogether, these results indicated that Mycoplasma hyopneumoniae interacted differently with H1N1 and H1N2 SIVs. In Mhp/H1N1 coinfection, synergistic effects were observed, with increased clinical signs and lung damages originating from both Mhp and H1N1 single infections. Thus, whereas H1N1 alone appeared less virulent than H1N2, the disease was more acute in MH1N1 group than in Mhp/H1N2 coinfected animals: the mean weight gain was more reduced, lesions of pneumonia were more extensive and microscopic lung lesions were more severe. These observations are typical of PRDC symptoms on field. By contrast, there were not apparent synergistic effects between the pathogens in Mhp/H1N2 coinfection, but rather the opposite, e.g. concurrent effect. The induction of immune response seemed earlier or more effective in MH1N2 group than in MH1N1. This could be linked to the difference also observed between responses that follow H1N2 and H1N1 single infections, respectively. Thus, it can be hypothesize that immune responses launched by H1N2 infection could play a role in the elimination of Mhp in the lung. The association between SIVs and Mhp are common in herds, even among healthy pigs that had no clinical symptoms (Palzer et al., 2008). The timing between the two infections but also the identity of the virus strain seems to have an important responsibility in the induction of clinical signs. All these results are in accordance with data we obtained from an epidemiological survey conducted in 125 herds affected by respiratory disorders, that showed a significant association between Mhp and H1N1, but not Mhp and H1N2, for the induction of enzootic pneumonia (Fablet et al., 2012).
Mechanisms responsible for the increased severity of clinical disease in case of dual infection are not clear and would require further investigations. It appears that cytokines produced locally at the site of infection have an important role in the development of the respiratory disease (Thanawongnuwech et al., 2004, Zhang et al., 2011). The pneumonia induced by Mhp is associated with a production of proinflammatory cytokines such as IL-1, IL-8 or TNF-α from 7 days to 28 days post inoculation (Redondo et al., 2009). The SIV infection also induces the production of IFN-α, TNF-α and IL-1, with a peak level at 1 day post-infection with SIV, what coincides with the onset of influenza symptoms and lung pathological changes (Barbe et al., 2011, Jo et al., 2007, Van Reeth et al., 1999). It can be hypothesized that an excessive and concurrent production of IFN-α, TNF-α and IL-1 in the lung would lead to significant tissue damages. Therefore, it would be interesting to investigate the modulations of cytokines and chemokines in Mhp/H1N1 and Mhp/H1N2 co-infected animals in order to better understand and compare these pathogen interactions. Another major contributor to the development of an increased pathology in dual infection is the fact that Mhp and SIV target both the epithelial cells and thereby cause damage to the mucociliary clearance mechanisms, which play an important role in pulmonary defense.
In conclusion, our findings indicated that experimental co-infection with Mycoplasma hyopneumoniae and European avian-like swine H1N1 is a suitable model for studying SIV-associated PRDC pathogenesis, for understanding mechanisms causing severe pneumonia and for testing intervention strategies for the control of the disease, also in order to reduce medication in pig herds. In addition, this experimental animal model could be useful for understanding mechanisms causing severe pneumonia in humans. Indeed, clinical manifestations and pathogenesis of influenza infections in pigs, which are physiologically, anatomically and immunologically similar to humans, closely resemble those observed in humans. In human, bacterial pneumonia after flu infection is often observed and a large majority of influenza complications or deaths results from secondary bacterial infection (Centers for Disease and Prevention, 2009, Morens et al., 2008).
Conflict of interest
None of the authors of this paper has financial or personal relationships with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors thank André Kéranflec’h, Gérard Bénévent, Pierre Ecobichon, Jean-Marie Guionnet and Nathalie Thomas for technical assistance. This work was supported by the “Conseil Régional de Bretagne” Fluporc Program (PRIR Bretagne 2183). The authors thank also the “Conseil Général 22” for the partial funding of the personnel of the production of decontaminated pigs and testing service.
References
- Barbe F., Atanasova K., Van Reeth K. Cytokines and acute phase proteins associated with acute swine influenza infection in pigs. Vet. J. 2011;187:48–53. doi: 10.1016/j.tvjl.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardini R., Leotti G., Nigrelli A.D., Rosignoli C., Foni E., Vila T., Joisel F., Galuppini A. Proceedings of 6th International Symposium on Emerging and Re-emerging Pig Diseases. 2011. Outbreak of H1N2 swine influenza in Italy: a field case; p. 268. [Google Scholar]
- Brons N., Neto R., Vila T., Pasini M., Joisel F. Proceedings of 6th International Symposium on Emerging and Re-emerging Pig Diseases. 2011. Outbreak of swine influenza, subtype H1N2: a case report and its financial consequences; p. 271. [Google Scholar]
- Centers for Disease and Prevention Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) – United States, May–August 2009. Morb. Mortal. Wkly. Rep. (MMWR) 2009;58:1071–1074. [PubMed] [Google Scholar]
- Choi Y.K., Goyal S.M., Joo H.S. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 2003;44:735–737. [PMC free article] [PubMed] [Google Scholar]
- Fablet C., Marois C., Simon G., Grasland B., Kobisch M., Jestin A., Madec F., Rose N. Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: a cross sectional study. Vet. Microbiol. 2012;157:152–163. doi: 10.1016/j.vetmic.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Haesebrouck F., Biront P., Pensaert M.B., Leunen J. Epizootics of respiratory tract disease in swine in Belgium due to H3N2 influenza virus and experimental reproduction of disease. Am. J. Vet. Res. 1985;46:1926–1928. [PubMed] [Google Scholar]
- Jo S.K., Kim H.S., Cho S.W., Seo S.H. Pathogenesis and inflammatory responses of swine H1N2 influenza viruses in pigs. Virus Res. 2007;129:64–70. doi: 10.1016/j.virusres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Kuntz-Simon G., Madec F. Genetic and antigenic evolution of swine influenza viruses in europe and evaluation of their zoonotic potential. Zoonoses Public Health. 2009;56:310–325. doi: 10.1111/j.1863-2378.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis C.S., Brown I.H., Foni E., Kuntz-Simon G., Maldonado J., Madec F., Essen S.C., Chiapponi C., Van Reeth K. Virological surveillance and preliminary antigenic characterization of influenza viruses in pigs in five European Countries from 2006 to 2008. Zoonoses Public Health. 2011;58:93–101. doi: 10.1111/j.1863-2378.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- Lanza I., Brown I.H., Paton D.J. Pathogenicity of concurrent infection of pigs with porcine respiratory coronavirus and swine influenza virus. Res. Vet. Sci. 1992;53:309–314. doi: 10.1016/0034-5288(92)90131-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving C.L., Brockmeier S.L., Vincent A.L., Palmer M.V., Sacco R.E., Nicholson T.L. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 2010;49:237–245. doi: 10.1016/j.micpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Madec F., Kobisch M. Bilan lésionnel des poumons de porcs charcutiers à l’abattoir. Journées de la Recherche Porcine en France. 1982;14:405–412. [Google Scholar]
- Maes L., Haesebrouck F., Pensaert M. Proceedings 8th International Pig Veterinary Society Congress. 1984. Experimental reproduction of clinical disease by intratracheal inoculation of fattening pigs with Swine Influenza Virus isolates; p. 60. [Google Scholar]
- Marois C., Dory D., Fablet C., Madec F., Kobisch M. Development of a quantitative Real-Time TaqMan PCR assay for determination of the minimal dose of Mycoplasma hyopneumoniae strain 116 required to induce pneumonia in SPF pigs. J. Appl. Microbiol. 2010;108:1523–1533. doi: 10.1111/j.1365-2672.2009.04556.x. [DOI] [PubMed] [Google Scholar]
- Marois C., Le Carrou J., Kobisch M., Gautier-Bouchardon A.V. Isolation of Mycoplasma hyopneumoniae from different sampling sites in experimentally infected and contact SPF piglets. Vet. Microbiol. 2007;120:96–104. doi: 10.1016/j.vetmic.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . Manual of Diagnostic Tests & Vaccines for Terrestrial Animals. 2008. Swine influenza. pp. 1236–1247. [Google Scholar]
- Olsen C.W., Brown I.H., Easterday B.C., van Reeth K. Swine influenza. In: Straw B.E.Z.J., d’Allaire S., Taylor D.J., editors. Disease of Swine. Blackwell Publishing; Oxford: 2006. pp. 469–482. [Google Scholar]
- Palzer A., Ritzmann M., Wolf G., Heinritzi K. Associations between pathogens in healthy pigs and pigs with pneumonia. Vet. Rec. 2008;162:267–271. doi: 10.1136/vr.162.9.267. [DOI] [PubMed] [Google Scholar]
- Redondo E., Masot A.J., Fernandez A., Gazquez A. Histopathological and immunohistochemical findings in the lungs of pigs infected experimentally with Mycoplasma hyopneumoniae. J. Comp. Pathol. 2009;140:260–270. doi: 10.1016/j.jcpa.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Sorensen V., Jorsal S.E., Mousing J. Disease of the respiratory system. In: Straw B.E.Z.J., D’Allaire S., Taylor D.J., editors. Blackwell Publishing; Oxford: 2006. pp. 149–177. (Diseases of Swine). [Google Scholar]
- Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L., Lohman K., Daum L.T., Suarez D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker E.L. Porcine respiratory disease complex – what is it and why does it remain a problem? Pig J. 2001:66–70. [Google Scholar]
- Thacker E.L. Mycoplasmal disease. In: Straw B.E.Z.J., D’Allaire S., Taylor D.J., editors. Disease of Swine. Blackwell Publishing; 2006. pp. 701–718. [Google Scholar]
- Thacker E.L., Thacker B.J., Janke B.H. Interaction between Mycoplasma hyopneumoniae and swine influenza virus. J. Clin. Microbiol. 2001;39:2525–2530. doi: 10.1128/JCM.39.7.2525-2530.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawongnuwech R., Thacker B., Halbur P., Thacker E.L. Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin. Diagn. Lab. Immunol. 2004;11:901–908. doi: 10.1128/CDLI.11.5.901-908.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Nauwynck H., Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res. Vet. Sci. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Labarque G., Pensaert M. Serological profiles after consecutive experimental infections of pigs with European H1N1, H3N2, and H1N2 swine influenza viruses. Viral Immunol. 2006;19:373–382. doi: 10.1089/vim.2006.19.373. [DOI] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H., Pensaert M. Dual infections of feeder pigs with porcine reproductive and respiratory syndrome virus followed by porcine respiratory coronavirus or swine influenza virus: a clinical and virological study. Vet. Microbiol. 1996;48:325–335. doi: 10.1016/0378-1135(95)00145-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Nauwynck H., Pensaert M. Clinical effects of experimental dual infections with porcine reproductive and respiratory syndrome virus followed by swine influenza virus in conventional and colostrum-deprived pigs. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2001;48:283–292. doi: 10.1046/j.1439-0450.2001.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K., Pensaert M.B. Porcine respiratory coronavirus-mediated interference against influenza virus replication in the respiratory tract of feeder pigs. Am. J. Vet. Res. 1994;55:1275–1281. [PubMed] [Google Scholar]
- Wei H., Lenz S.D., Van Alstine W.G., Stevenson G.W., Langohr I.M., Pogranichniy R.M. Infection of cesarean-derived colostrum-deprived pigs with porcine circovirus type 2 and Swine influenza virus. Comp. Med. 2010;60:45–50. [PMC free article] [PubMed] [Google Scholar]
- Yazawa S., Okada M., Ono M., Fujii S., Okuda Y., Shibata I., Kida H. Experimental dual infection of pigs with an H1N1 swine influenza virus (A/Sw/Hok/2/81) and Mycoplasma hyopneumoniae. Vet. Microbiol. 2004;98:221–228. doi: 10.1016/j.vetmic.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Zhang H., Lunney J.K., Baker R.B., Opriessnig T. Cytokine and chemokine mRNA expression profiles in tracheobronchial lymph nodes from pigs singularly infected or coinfected with porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (MHYO) Vet. Immunol. Immunopathol. 2011;140:152–158. doi: 10.1016/j.vetimm.2010.11.019. [DOI] [PubMed] [Google Scholar]


