Abstract
Flavivirus non-structural protein 4A (NS4A) induces membrane rearrangements to form viral replication complex and functions as interferon antagonist. However, other non-structural roles of NS4A protein in relation to virus life-cycle are poorly defined. This study elucidated if dengue virus (DENV) NS4A protein interacts with host proteins and contributes to viral pathogenesis by screening human liver cDNA yeast-two-hybrid library. Our study identified polypyrimidine tract-binding protein (PTB) as a novel interacting partner of DENV NS4A protein. We reported for the first time that PTB influenced DENV production. Gene-silencing studies showed that PTB did not have an effect on DENV entry and DENV RNA translation. Further functional studies revealed that PTB influenced DENV production by modulating negative strand RNA synthesis. This is the first study that enlightens the interaction of DENV NS4A protein with PTB, in addition to demonstrating the novel role of PTB in relation to mosquito-borne flavivirus life-cycle.
Keywords: Dengue virus, NS4A, PTB, RNA synthesis
Introduction
Dengue virus (DENV) is a single-stranded RNA virus belonging to Flaviviridae family. There are four DENV serotypes that are antigenically different, namely DENV1, DENV2, DENV3 and DENV4. Its RNA genome encodes a single polypeptide which is subsequently processed by virus and host proteases to form three structural and seven non-structural proteins in the order C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5 [1], [2]. The capsid protein undergoes oligomerization and interacts with viral RNA to form nucleocapsid. The pre-membrane (prM) and envelope (E) proteins synthesized at endoplasmic reticulum form heterodimers and further interact with virus nucleocapsid to generate immature virus particles. The immature virus particles are transported through trans-Golgi network and cleaved by host protease furin to form infectious mature virus particles. Mature virions are subsequently released from the host cell by exocytosis [3], [4].
Non-structural (NS) proteins play an essential role in flavivirus replication. NS1 protein is involved in viral RNA replication, signal transduction and complement activation [5], [6], [7], [8], [9]. NS2A is involved in generation of virus-induced membranes during virus assembly [10]. NS2B acts as the co-factor for the protease activity of NS3 protein [11], [12]. NS3 functions as a serine protease/RNA helicase [13]. NS4A induces membrane rearrangements to form viral replication complex and acts as an interferon antagonist [14], [15]. NS4B inhibits type I interferon response of host cells and modulates viral replication through its association with NS3 [15], [16]. NS5 protein possesses methyl transferase activity and RNA-dependent RNA polymerase activity [13]. However, the exact molecular mechanism underlying the functions of these viral proteins remains to be deciphered in great detail.
In this study, we aimed to understand the role of NS4A protein in relation to DENV life-cycle and found polypyrimidine tract-binding protein (PTB) as a novel interacting partner of NS4A protein. Functional studies revealed that PTB influenced DENV negative strand RNA synthesis by binding with DENV RNA genome. This is the first study reporting the association of DENV NS4A protein with PTB and sheds light on the biological significance of this binding in relation to DENV replication.
Materials and methods
Cells and viruses. Huh-7 cells were maintained in Dulbecco’s Modified Eagle medium (Sigma) containing 10% fetal calf serum (Invitrogen) and incubated at 37 °C with 5% CO2. Dengue 2 virus (New Guinea C) propagated in C6/36 cells was used throughout this study. Huh-7 cells were infected with DENV at MOI of 0.1.
Plasmid construction. Full-length DENV NS4A was cloned into pGBKT7 vector (Clontech) using NdeI and BamHI restriction enzyme sites. The resulting constructs were sequenced to ensure correct reading frames and were subsequently used as bait in yeast-two-hybrid (Y2H) library screening. The short hairpin RNA (shRNA) (5′-AAGGAACTTCCATCATTCCAGAGAACTTGCTTCTTCTCTGGAATGATGGAAGTTCCTATAGTGA-3′) directed against human PTB mRNA was cloned into psiRNAhH1-GFPzeo vector to generate psiRNA-PTB. Huh-7 cells were transfected with psiRNA-PTB using lipofectamine 2000. Stable transfectants were selected with zeocin (200 μg/ml, Invitrogen) and called PTBKD cells. Huh-7 cells transfected with scrambled shRNA sequence produced scrambledKD cells.
Y2H screening. Y2H mating assays were carried out using NS4A as bait and pre-transformed human liver cDNA yeast library (Clontech) as described by the manufacturer. The resulting positive colonies were picked to isolate the library plasmids using Yeast plasmid extraction kit (Clontech) and sequenced for BLAST analysis (GenBank).
Co-immunoprecipitaion. Huh-7 cells (5 × 105) were infected with DENV. At 24 h post-infection, cell lysate was pre-mixed with 2 μg of anti-DENV NS4A/anti-PTB conjugated magnetic microbeads and purified using μMACs column (Miltenyi Biotec). Samples obtained from co-immunoprecipitation assays or whole cell lysates were subjected to Western blotting using anti-DENV NS4A (1:400)/anti-PTB (1:400) antibodies.
Localization studies. Huh-7 cells were infected with DENV. At 24 h post-infection, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X. The anti-NS4A (1:400) or anti-PTB (1:700) antibodies were used as primary antibodies and secondary goat antibodies conjugated to FITC or Texas red (Chemicon) were used. The nuclei were stained with DAPI (Molecular Probes). The specimens were viewed with a laser scanning confocal inverted microscope (Leica TCS SP2).
Virus growth kinetics. Huh-7, scrambledKD and PTBKD cells were infected with DENV or transfected with RNA in vitro transcribed from full length infectious clone of DENV. At the indicated timings, culture supernatants were collected for plaque assay.
Real-time RT-PCR. Huh-7, scrambledKD and PTBKD cells were infected with DENV. Total RNA was extracted using TRIzol reagent (Invitrogen) and subjected to real-time RT-PCR using iTaq™ Supermix (BIO-RAD) with primer sequences targeting NS1 region. The forward primer is 6-carboxyfluorescein-labeled 5′-CACAACCATGAAGAGGGCATTTG-FAM-G-3′ and the reverse primer is unlabeled NS1 reverse primer, 5′-TTTGTTTCCACATCAGATTCCCA-3′[17]. Reactions were performed using Applied Biosystems 7500 real-time PCR system. Standard curve was generated using DENV RNA isolated from known quantity of virus. The amount of total viral RNA from PTBKD or scrambledKD cells was determined relative to that from control Huh-7 cells.
Detection of PTB-DENV association. Huh-7, scrambledKD and PTBKD cells were infected with DENV or transfected with biotinylated RNA in vitro transcribed from full length infectious clone of DENV. After 14 h, cells were cross-linked with 1% formaldehyde and lysed for co-immunoprecipitation or pull down assay using anti-PTB Ab or Streptavidin beads (Invitrogen). Real-time RT-PCR was performed on the RNA isolated from anti-PTB Ab immunoprecipitated sample. The samples obtained from pull down assays were analyzed by Western blotting using anti-PTB and anti-NS4A Ab.
Results
Identification of PTB as a binding partner of NS4A protein
Using DENV NS4A protein as bait, Y2H screening was performed against human liver cDNA library. Five independent clones that encoded PTB protein were found to interact with NS4A protein. To confirm NS4A-PTB association in mammalian system, co-immunoprecipitation was performed. Huh-7 cells were infected with DENV. At 24 h p.i., cell lysates were immunoprecipitated with anti-PTB Ab followed by immunoblotting using anti-DENV NS4A Ab. As shown in Fig. 1 A(i), immuno-reactive bands were observed only with DENV-infected Huh-7 cells (Lane 3). The reciprocal co-immunoprecipitation performed showed consistent results [Fig. 1A(ii)]. The precipitation controls were included to ensure successful precipitation in all groups [Fig. 1A(iii & iv)]. The expression of NS4A and PTB proteins was shown in Fig. 1A(v & vi) in whole cell lysates (WCL). Co-immunoprecipitation was also performed using mouse/rabbit isotype control Abs to eliminate the possibility of non-specific precipitation [Fig. 1A(vii & viii)]. This demonstrated for the first time that DENV NS4A protein interacted specifically with PTB.
Fig. 1.

PTB-NS4A interaction during DENV infection. (A) (i) Huh-7 cells were infected with DENV and immunoprecipitated with anti-PTB Ab. Presence of band in Lane 3 confirmed NS4A-PTB binding in Huh-7 cells. (ii) Reciprocal Co-IP performed using anti-NS4A Ab. (iii–viii) Immunoprecipitation controls, input controls and isotype Ab controls. (B) Confocal microscopy showing the co-localization of NS4A and PTB in Huh-7 cells which further confirmed the interplay between NS4A and PTB.
Co-localization of DENV NS4A and PTB
Further investigation was carried out to determine the cellular localization of DENV NS4A and PTB using confocal microscopy. PTB was found in the nucleus and cytoplasm of both mock- [Fig. 1B(i)] and DENV-infected cells [Fig. 1B(v)]. NS4A protein was detected mainly in the perinuclear regions although nuclear staining was also observed to a lesser extent [Fig. 1B(vi)]. Yellow fluorescence observed with the overlay image [Fig. 1B(viii)] confirmed the co-localization of DENV NS4A protein with PTB in DENV-infected cells. This further demonstrated that the interaction between NS4A and PTB is genuine.
PTB influences DENV production
To examine the role of PTB in relation to virus production, PTB knock-down (PTBKD) Huh-7 cells were generated. The effect of gene silencing in Huh-7 cells was confirmed by Western blotting and densitometry (SynTools from SynGene). The psiRNA-PTB mediated gene knock-down decreased the endogenous PTB level by 60% as revealed by densitometry (Fig. 2 A). The effect of PTBKD on DENV production was examined in Huh-7 cells by infecting scrambledKD and PTBKD cells with DENV. At the indicated time points, culture supernatants were collected for plaque assay. Reduced virus production was observed with DENV-infected PTBKD cells (Fig. 2B) at as early as 1 day post-infection although 2–3 log units reduction in virus titres were obtained at 2 and 3 days post-infection (P < 0.05). This indicated that PTB influenced DENV production.
Fig. 2.

PTB influences DENV production. (A) Western blot analysis showing the effect of PTB gene silencing in Huh-7 cells. (B) Huh-7, scrambledKD and PTBKD cells were infected with DENV and supernatants harvested at indicated timings were subjected to plaque assay. Reduced virus titres were observed with PTBKD cells compared to scrambledKD/Huh-7 cells. The results represented the mean activity of 3 independent experiments ± standard deviation. The P-values were calculated using student’s t test (P < 0.05). (C) Virus titres were determined from Huh-7, scrambledKD and PTBKD cells transfected with DENV RNA. Virus titres were reduced in PTBKD cells.
Early events in DENV life-cycle does not influence DENV production
To test if PTB-induced changes in virus titres were due to enhanced virus entry and genome uncoating, these processes were bypassed by transfecting scrambledKD and PTBKD cells with RNA in vitro transcribed from full length infectious clone of DENV. At the indicated time points, culture supernatants were collected for plaque assay. Virus titres were significantly reduced (P < 0.05) by 2–3 log units in PTBKD cells transfected with full length DENV RNA compared to scrambledKD cells (Fig. 2C). This indicated that PTB influenced virus titres following genome uncoating.
PTB influences viral RNA synthesis
To examine the effect of PTB on viral RNA synthesis, Huh-7, scrambledKD and PTBKD cells were infected with DENV. Total RNA was extracted and subjected to real-time RT-PCR. Viral RNA levels in DENV-infected PTBKD cells were significantly reduced (40%, P < 0.05) compared to DENV-infected scrambledKD/control Huh-7 cells (Fig. 3 ). This demonstrated that PTB influenced DENV RNA synthesis.
Fig. 3.

Effect of PTB on viral RNA synthesis. Real-time RT-PCR was performed on the RNAs extracted from DENV-infected Huh-7, scrambledKD and PTBKD cells. Significant reduction (P < 0.05) in viral RNA levels were observed.
PTB associates with DENV RNA
Huh-7, scrambledKD and PTBKD cells were infected with DENV. After cross-linking, cells were co-immunoprecipitated using anti-PTB Ab. Real-time RT-PCR was performed on the RNA isolated from anti-PTB Ab immunoprecipitated sample. As shown in Fig. 4 A, PTB-bound DENV RNA levels were lower by 40% in PTBKD cells compared to scrambledKD cells after normalization against actin control. This indicated that PTB binding to DENV RNA is significantly impaired in PTBKD cells.
Fig. 4.
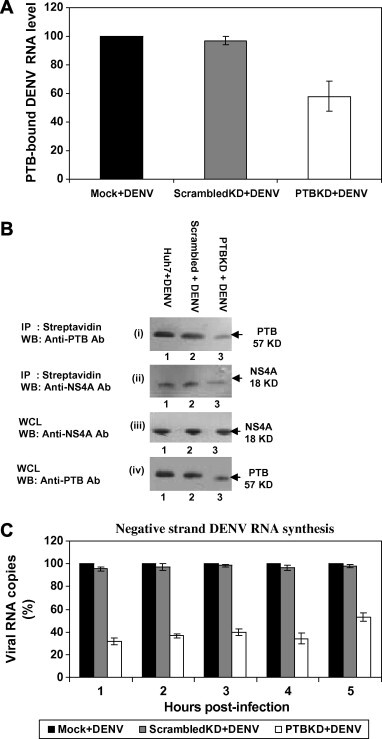
PTB binding to DENV RNA genome and negative strand RNA synthesis. (A) DENV-infected Huh-7, scrambledKD and PTBKD cells were co-immunoprecipitated using anti-PTB Ab. Real-time RT-PCR was performed on the RNA isolated from anti-PTB Ab immunoprecipitated sample. Reduction in PTB-bound DENV RNA levels were observed in PTBKD cells compared to scrambledKD cells (P < 0.05) after normalization against actin control. (B) Huh-7, scrambledKD and PTBKD cells were transfected with biotinylated DENV RNA and pull down assay was performed using streptavidin dynabeads followed by Western blotting using anti-PTB/anti-NS4A Ab. Lower intensity of PTB or NS4A bands was observed in PTBKD cells (Lane 3) compared to control/scrambledKD cells (Lanes 1 and 2). (C) T7-tagged real-time RT-PCR was performed on the RNAs extracted from DENV-infected Huh-7, scrambledKD and PTBKD cells. Significant reduction (P < 0.05) in DENV negative strand RNA levels were observed.
Huh-7, scrambledKD and PTBKD cells were transfected with biotinylated RNA and pull down assay was performed using streptavidin dynabeads followed by Western blotting using anti-PTB/anti-NS4A Ab. Interestingly, the intensity of PTB or NS4A bands were lower (20–30% lower as revealed by densitometry analysis of the immunoblot) in PTBKD cells [Fig. 4B(i & ii), Lane 3] compared to control/scrambledKD cells [Fig. 4B(i & ii), Lanes 1 & 2]. These results confirmed that PTB associates with DENV RNA and NS4A during DENV infection.
Influence on DENV negative strand RNA synthesis
Davis and colleagues [18] showed that binding of elongation factor 1 alpha to West Nile virus RNA influences negative strand RNA synthesis. PTB was shown to bind DENV4 RNA. We thus investigated if DENV negative strand synthesis is affected when PTB level is lowered by gene silencing. Total RNA was extracted from DENV-infected Huh-7, scrambledKD and PTBKD cells. Negative strand RNA levels were measured using T7-tagged real-time RT-PCR as described by Davis and colleagues [18]. As shown in Fig. 4C, DENV negative strand RNA levels in DENV-infected PTBKD cells were approximately 65% reduced (P < 0.05) compared to DENV-infected scrambledKD/control Huh-7 cells. This indicated that efficient binding of PTB with DENV RNA is essential for DENV negative strand RNA synthesis.
Discussion
Dengue viruses are the most important human arboviral pathogens of greatest global public health importance. DENV infection causes a broad spectrum of clinical manifestations ranging from asymptomatic fever to potentially fatal hemorrhagic manifestations. Approximately 2.5 billion people living in tropical and subtropical countries are at risk of epidemic transmission and 50–100 million-dengue infections occur annually. About 500,000 dengue-infected individuals develop the life threatening Dengue Hemorrhagic Fever and Dengue Shock Syndrome [19], [20]. There is no approved vaccine or antiviral therapies available against DENV infections. The only form of treatment available today is the supportive treatment and prevention is through vector control and boosting the immunity. Hence there is a great urge for the development of vaccines or novel antivirals. Viruses are known to subvert host proteins to create a more favorable environment for their replication. It is thus important to understand the virus-host protein interactions that mediate different steps of DENV life cycle to develop good antiviral drugs.
In this study, DENV NS4A protein, a component of viral replicative machinery was used to elucidate the biological role of NS4A protein in DENV pathogenesis. Human liver cDNA library was screened using DENV NS4A protein as bait. We demonstrated for the first time that DENV NS4A protein interacted with PTB. PTB is a member of heterogenous nuclear ribonucleoprotein that binds to cellular and viral RNA [21]. PTB has been implicated in multiple steps of pre-mRNA processing [22], polyadenylation regulation [23] and viral/cellular IRES-dependent translation of RNA [24], [25], [26], [27]. PTB has been shown to regulate RNA transcription, translation and virus propagation of several viruses including picornavirus, coronavirus and herpes virus [28], [29], [30]. The involvement of PTB in regulating viral replication and translation was also shown in Hepatitis C virus [31], [32], [33], [34]. Binding of PTB to untranslated regions of mosquito-borne flaviviruses such as Japanese encephalitis virus and Dengue 4 virus were also reported although the biological significance of this binding has not been demonstrated [35], [36].
This study identified the novel interaction between DENV NS4A protein and PTB. We have analyzed the importance of NS4A-PTB association using a combination of biochemical and molecular techniques and demonstrated that PTB influenced DENV production (Fig. 2A). RNA viruses are known to replicate in the cytoplasm of infected cells although Uchil and colleagues [37] reported that DENV replication occured within the nucleus. Moreover, several structural and non-structural proteins of flavivirus were shown to enter the nucleus and this nuclear phase is critical for their replication. Though PTB is present predominantly in the nucleus at static state, a significant cytoplasmic localization was also reported and it can shuttle between the nucleus and cytoplasm [38], [39]. Thus the cellular distribution of PTB together with its interaction with a component of DENV replicative complex indicated that PTB can influence DENV production.
Next, we examined the molecular mechanism underlying this PTB-induced changes in DENV production and showed that they were independent of virus entry, genome uncoating and protein translation (Fig. 2C and Supplementary Fig. 1). This study identified that PTB binding to DENV RNA genome is crucial and reduced binding affected negative strand RNA synthesis which accounted for the altered DENV production in PTBKD cells (Fig. 4). This is in consistent with Mouse Hepatitis virus where PTB influenced transcription, but not translation of viral RNA [40]. Recently, Davis and colleagues [18] showed that binding of elongation factor 1 alpha to West Nile virus RNA genome is critical for viral negative strand RNA synthesis. This suggested that PTB might play a similar role in DENV replication or PTB might function together with elongation factor 1 alpha to facilitate DENV negative strand RNA synthesis.
In conclusion, our study identified a novel interaction between DENV NS4A, a component of viral replicative machinery and PTB and revealed the biological significance of this association in relation to virus life-cycle. This study broadened our current understanding on the functions of DENV NS4A protein besides revealing the role of PTB in DENV replication. Moreover, this study identified a new anti-DENV target (PTB) and formed the platform towards the development of new anti-DENV drugs.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2009.05.036.
Appendix A. Supplementary data
Supplementary Fig. 1.

Effect of PTB on viral RNA translation. To examine if PTB-induced changes in virus titres were due to changes in viral RNA translation, luciferase reporter assay was performed. Huh-7, scrambledKD and PTBKD cells were transfected with RNAs in vitro transcribed from DENV reporter construct containing the luciferase gene flanked by 5′ UTR and 3′ UTR of DENV and control actin construct containing the luciferase gene flanked by 5′ UTR of actin and 3′ poly A tail. After 24 h post-transfection, cells were lysed and subjected to luciferase assay (Promega). Luciferase activity was not significantly changed (P > 0.05) in DENV reporter construct RNA-transfected Huh-7, scrambledKD and PTBKD cells after normalization against control actin construct. This showed that PTB did not influence viral RNA translation.
References
- 1.Rice C.M., Lenches E.M., Eddy S.R., Shin S.J., Sheets R.L., Strauss J.H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229:726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 2.Chambers T.J., Hahn C.S., Galler R., Rice C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Corver J., Chipman P.R., Zhang W., Pletnev S.V., Sedlak D., Baker T.S., Strauss J.H., Kuhn R.J., Rossmann M.G. Structures of immature flavivirus particles. EMBO J. 2004;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindenbach B.D., Rice C.M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackenzie J.M., Jones M.K., Young P.R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs M.G., Robinson P.J., Bletchly C., Mackenzie J.M., Young P.R. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 2000;14:1603–1610. doi: 10.1096/fj.14.11.1603. [DOI] [PubMed] [Google Scholar]
- 8.Avirutnan P., Punyadee N., Noisakran S., Komoltri C., Thiemmeca S., Auethavornanan K., Jairungsr A., Kanlaya R., Tangthawornchaikul N., Puttikhunt C., Pattanakitsakul S.N., Yenchitsomanus P.T., Mongkolsapaya J., Kasinrerk W., Sittisombut N., Husmann M., Blettner M., Vasanawathana S., Bhakdi S., Malasit P. Vascular leakage in severe Dengue virus infections: a potential role for the non-structural viral protein NS1 and complement. J. Infect. Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 9.Muylaert I.R., Galler R., Rice C.M. Genetic analysis of the yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J. Virol. 1997;71:291–298. doi: 10.1128/jvi.71.1.291-298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung J.Y., Pijlman G.P., Kondratieva N., Hyde J., Mackenzie J.M., Khromykh A.A. Role of nonstructural protein NS2A in flavivirus assembly. J. Virol. 2008;82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falgout B., Pethel M., Zhang Y.M., Lai C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung D., Schroder K., White H., Fang N.X., Stoermer M.J., Abbenante G., Martin J.L., Young P.R., Fairlie D.P. Activity of recombinant dengue 2 virus NS3 protease in the presence of a truncated NS2B co-factor, small peptide substrates, and inhibitors. J. Biol. Chem. 2001;276:45762–45771. doi: 10.1074/jbc.M107360200. [DOI] [PubMed] [Google Scholar]
- 13.Perera R., Kuhn R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008;11:369–377. doi: 10.1016/j.mib.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller S., Kastner S., Krijnse-Locker J., Buhler S., Bartenschlager R. The nonstructural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Jordan J.L., Laurent-Rolle M., Ashour J., Martinez-Sobrido L., Ashok M., Lipkin W.I., Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umareddy I., Chao A., Sampath A., Gu F., Vasudevan G. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 2006;87:2605–2614. doi: 10.1099/vir.0.81844-0. [DOI] [PubMed] [Google Scholar]
- 17.Helt A.M., Harris E. S-phase-dependent enhancement of dengue virus 2 replication in mosquito cells, but not in human cells. J. Virol. 2005;79:13218–13230. doi: 10.1128/JVI.79.21.13218-13230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis W.G., Blackwell J.L., Shi P.Y., Brinton M.A. Interaction between the cellular protein eEF1A and the 3′-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J. Virol. 2007;81:10172–10187. doi: 10.1128/JVI.00531-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halstead S.B. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 20.Leong A.S., Wong K.T., Leong T.Y., Tan P.H., Wannakrairot P. The pathology of dengue hemorrhagic fever. Semin. Diagn. Pathol. 2007;24:227–236. doi: 10.1053/j.semdp.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ghetti A., Pinol-Roma S., Michael W.M., Morandi C., Dreyfuss G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20:3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner E.J., Garcia-Blanco M.A. Polypyrimidine tract binding protein antagonizes exon definition. Mol. Cell. Biol. 2001;21:3281–3288. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira A., Takagaki Y., Brackenridge S., Wollerton M., Manley J.L., Proudfoot N.J. The upstream sequence element of the C2 complement poly(A) signal activates mRNA 3′ end formation by two distinct mechanisms. Genes Dev. 1998;12:2522–2534. doi: 10.1101/gad.12.16.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belsham G.J., Sonenberg N. RNA–protein interactions in regulation of picornavirus RNA translation. Microbiol. Rev. 1996;60:499–511. doi: 10.1128/mr.60.3.499-511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellen C.U., Pestova T.V., Litterst M., Wimmer E. The cellular polypeptide p57 (pyrimidine tract-binding protein) binds to multiple sites in the poliovirus 5′ nontranslated region. J. Virol. 1994;68:941–950. doi: 10.1128/jvi.68.2.941-950.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski A., Hunt S.L., Patton J.G., Jackson R.J. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y.K., Hahm B., Jang S.K. Polypyrimidine tract-binding protein inhibits translation of bip mRNA. J. Mol. Biol. 2000;304:119–133. doi: 10.1006/jmbi.2000.4179. [DOI] [PubMed] [Google Scholar]
- 28.Florez P.M., Sessions O.M., Wagner E.J., Gromeier M., Garcia-Blanco M.A. The polypyrimidine tract binding protein is required for efficient picornavirus gene expression and propagation. J. Virol. 2005;79:6172–6179. doi: 10.1128/JVI.79.10.6172-6179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S.T., Lai M.M. Viral and cellular proteins involved in coronavirus replication. Curr. Top. Microbiol. Immunol. 2005;287:95–131. doi: 10.1007/3-540-26765-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bieleski L., Hindley C., Talbot S.J. A polypyrimidine tract facilitates the expression of Kaposi’s sarcoma-associated herpesvirus vFLIP through an internal ribosome entry site. J. Gen. Virol. 2004;85:615–620. doi: 10.1099/vir.0.19733-0. [DOI] [PubMed] [Google Scholar]
- 31.Aizaki H., Choi K.S., Liu M., Li Y.J., Lai M.M. Polypyrimidine-tract-binding protein is a component of the HCV RNA replication complex and necessary for RNA synthesis. J. Biomed. Sci. 2006;13:469–480. doi: 10.1007/s11373-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 32.Chang K.S., Luo G. The polypyrimidine tract-binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res. 2006;115:1–8. doi: 10.1016/j.virusres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Tischendorf J.J., Beger C., Korf M., Manns M.P., Krüger M. Polypyrimidine tract-binding protein (PTB) inhibits Hepatitis C virus internal ribosome entry site (HCV IRES)-mediated translation, but does not affect HCV replication. Arch. Virol. 2004;149:1955–1970. doi: 10.1007/s00705-004-0341-8. [DOI] [PubMed] [Google Scholar]
- 34.Domitrovich A.M., Diebel K.W., Ali N., Sarker S., Siddiqui A. Role of La autoantigen and polypyrimidine tract-binding protein in HCV replication. Virology. 2005;335:72–86. doi: 10.1016/j.virol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.M., Jeong Y.S. Polypyrimidine tract-binding protein interacts with the 3′ stem-loop region of Japanese encephalitis virus negative-strand RNA. Virus Res. 2006;115:131–140. doi: 10.1016/j.virusres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 36.De Nova-Ocampo M., Villegas-Sepúlveda N., del Angel R.M. Translation elongation factor-1alpha, La, and PTB interact with the 3′ untranslated region of dengue 4 virus RNA. Virology. 2002;295:337–347. doi: 10.1006/viro.2002.1407. [DOI] [PubMed] [Google Scholar]
- 37.Uchil P.D., Kumar A.V., Satchidanandam V. Nuclear localization of flavivirus RNA synthesis in infected cells. J. Virol. 2006;80:5451–5464. doi: 10.1128/JVI.01982-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamath R.V., Leary D.J., Huang S. Nucleocytoplasmic shuttling of polypyrimidine tract-binding protein is uncoupled from RNA export. Mol. Biol. Cell. 2001;12:3808–3820. doi: 10.1091/mbc.12.12.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawicka K., Bushell M., Spriggs K.A., Willis A.E. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem. Soc. Trans. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 40.Choi K.S., Huang P., Lai M.M. Polypyrimidine-tract-binding protein affects transcription but not translation of mouse hepatitis virus RNA. Virology. 2002;303:58–68. doi: 10.1006/viro.2002.1675. [DOI] [PubMed] [Google Scholar]


