Fig. 1.
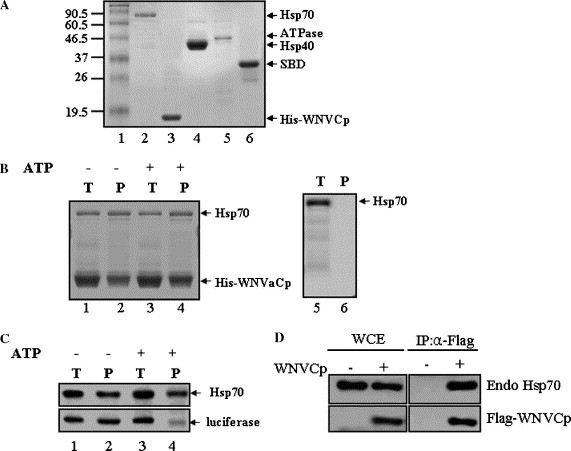
WNVCp forms a complex with Hsp70. (A) Recombinant proteins, Hsp70, His-WNVCp, Hsp40, ATPase domain, and SBD, were purified and analyzed by SDS–PAGE and visualized with Coomassie blue. (B) Reaction mixtures containing Hsp70 (0.5 μM) + His-WNVCp (4 μM) (lanes 1–4) or Hsp70 (1 μM) alone (lanes 5 and 6) were incubated (T: total, lanes 1, 3, and 5) in the absence (lanes 1, 2, 5, and 6) or presence (lanes 3 and 4) of ATP, and protein complexes were precipitated (P: precipitated, lanes 2, 4, and 6) by Ni2+–agarose. Precipitated proteins were analyzed by SDS–PAGE followed by Coomassie blue staining. (C) Reaction mixtures containing Hsp70 (0.5 μM) and luciferase that had been chemically denatured by guanidium–HCl (0.5 μM) (lanes 1–4) were incubated (T: total, lanes 1 and 3) in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of ATP, and protein complexes were precipitated (P: precipitated, lanes 2, 4, and 6) by Hsp70 antibody (5a5). Precipitated proteins were analyzed by SDS–PAGE followed by Western blotting using mono-Hsp70 mouse (5a5) and poly-luciferase rabbit (L0159) antibodies. (D) The plasmid expressing HA-WNVCp was transfected into 293T cells. The cell lysates were immunoprecipitated using anti-HA mouse antibodies. The whole cell extract (WCE) and immunoprecipitates (IP) were detected using anti-HA rabbit and anti-Hsp70 mouse antibodies.
