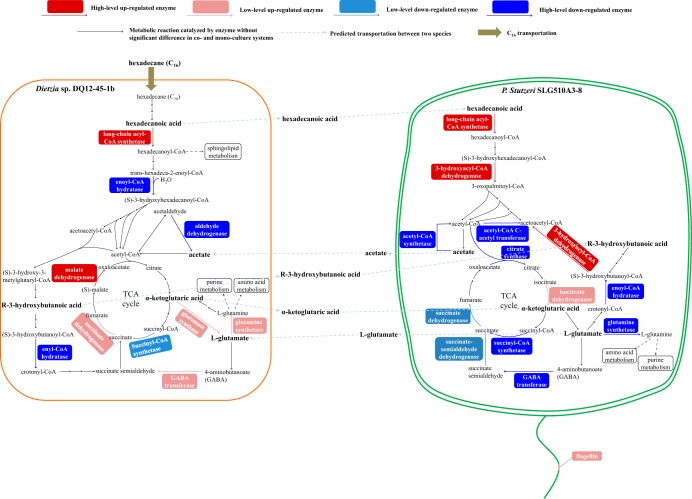
FIG 5.
Framework showing the regulation of expression of proteins relating to hexadecanoic acid, 3-hydroxybutanoic acid, α-ketoglutaric acid, acetate, and l-glutamate metabolic pathways in Dietzia sp. DQ12-45-1b and P. stutzeri SLG510A3-8 in the artificial consortium when they were exposed to C16. C16 was consumed by Dietzia sp. DQ12-45-1b and was converted to hexadecanoic acid and then to β-oxidation products. Meanwhile, two P. stutzeri enzymes involved in hexadecanoic acid β-oxidation, the long-chain acyl coenzyme A (acyl-CoA) synthetase and the 3-hydroxyacyl-CoA dehydrogenase, had high-level upregulation in the coculture system, indicating that hexadecanoic acid produced by Dietzia sp. was assimilated by P. stutzeri in the coculture system. The higher expression of P. stutzeri enzymes involved in hexadecanoic acid β-oxidation and the high-level downexpression of three P. stutzeri enzymes involved in acetate consumption, acetyl-CoA synthetase, acetyl-CoA C-acetyltransferase, and citrate synthase, would lead to the increased production of acetate. Coincidentally, the Dietzia sp. enzyme aldehyde dehydrogenase responding for acetate production was high-level downregulated when the cells coexisted with P. stutzeri. The lower expression of this protein could be interpreted as if Dietzia sp. indeed utilized extracellular acetate produced by its partner. If this was true, R-3-hydroxybutanoate, a downstream product of acetate, might accumulate in Dietzia sp. and then be secreted to the surrounding environment for P. stutzeri utilization. This inference was confirmed by the fact that an enoyl-CoA hydratase in P. stutzeri, whose function was the hydrolysis of crotonyl-CoA to the R-3-hydroxybutanoate derivative (S)-3-hydroxybutanoyl-CoA, was high-level downregulated in the coculture system, while a P. stutzeri 3-hydroxyacyl-CoA dehydrogenase, whose function was the dehydrogenation of (S)-3-hydroxybutanoyl-CoA to acetoacetyl-CoA, was high-level upregulated. In addition, if acetate was supplied by P. stutzeri to Dietzia sp., α-ketoglutaric acid, a second downstream product of acetate, might accumulate in Dietzia sp. and then be converted to l-glutamate in Dietzia sp. or be secreted to the surrounding environment for P. stutzeri utilization. This inference was confirmed by the facts that the expression of succinyl-CoA synthetase in Dietzia, which used the downstream product of α-ketoglutaric acid as the substrate, was low-level downregulated, while the expression of Dietzia glutamate synthase, which catalyzed l-glutamate production from α-ketoglutaric acid, was low-level upregulated. In P. stutzeri, the high-level downregulation of enzymes relating to l-glutamate consumption, such as glutamine synthetase and gamma-aminobutyric acid (GABA) transferase, suggested that l-glutamate might be accumulated and then be secreted to the surrounding environment for Dietzia sp. utilization. This hypothesis could explain why the glutamine synthetase and GABA transferase in Dietzia sp. were low-level upregulated in the coculture system.