Figure 1:
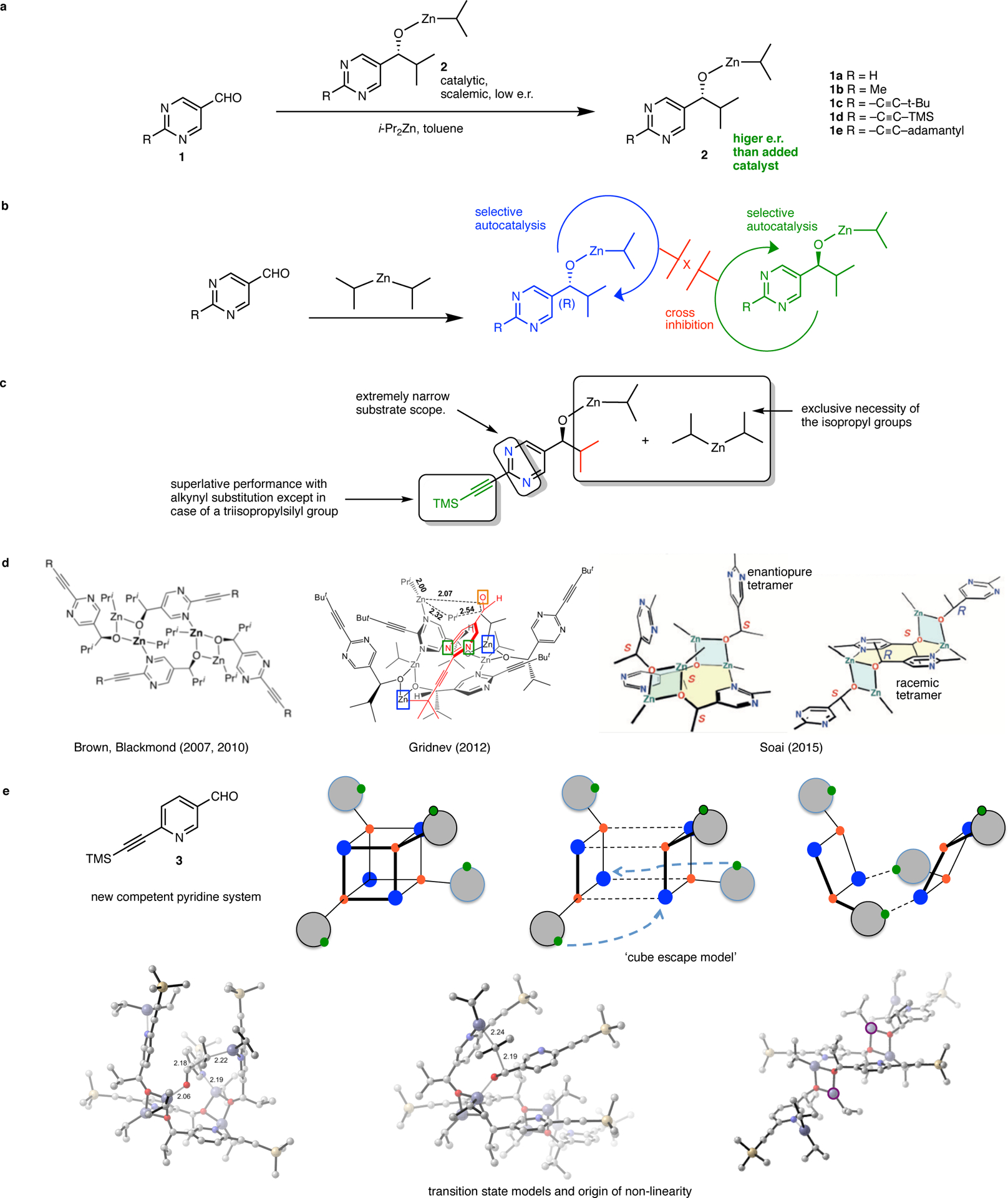
The Soai reaction system, current mechanistic understanding and salient contributions from this work. a. The diisopropylzinc alkylation of pyrimidine-5-carbaldehydes, termed the ‘Soai reaction’ is characterized by highly efficient autocatalysis and chiral amplification. The product zinc alkoxide is a selective autocatalyst for its own formation. Following Soai’s pioneering report with substrate 1a, competent activity has been subsequently described for other derivatives. In particular, the akynyl-substituted substrates 1c-1e demonstrate superior reactivity and chiral amplification. The transformation is exquisitely sensitive to initial chiral imbalances including perturbations caused by isotopic chirality. The reaction demonstrates spontaneous symmetry breaking and qualifies as an example of absolute asymmetric synthesis. b. Overall kinetic scheme for amplifying asymmetric autocatalysis (Frank, 1953). Accordingly, the product alkoxide enantiomers are self-catalytic while also being mutually inhibitory. Outstanding questions regarding the molecular details include the identity and mode of operation of the autocatalyst, a compelling transition-state hypothesis, an explanation for the origin of the non-linear effect, and a justification for the puzzling, idiosyncratic substrate constraints. c. Poorly understood structural constraints in the Soai reaction. d. The state of the art in mechanistic elucidation. From left – the SMS tetramer connectivity as first described by Brown and Blackmond, the Gridnev TS model of catalysis by the SMS tetramer and, crystal structures of the homochiral and heterochiral SMS tetramers. The crystal structure depicts the homochiral tetramer as a bowl shaped assembly with a central macrocycle (shaded yellow) and two ‘pyrimidine arms’ oriented in the same direction (in this case, the top face). The heterochiral tetramer possesses an identical connectivity but with the arms oriented on opposing faces of the central macrocycle. e. Contributions from this work include the discovery of a new amplifying autocatalytic pyridine system that leads to a structural simplification, the ‘cube escape’ model which elucidates the structural logic for the evolution of the Soai tetramer and the presentation of a new floor-to-floor TS model, fundamentally distinct from the Gridnev proposal, based on experimental and computational studies. The new model also provides insights into the structural constraints of the reactants and a compelling explanation for the origin of non-linearity.
