Figure 3:
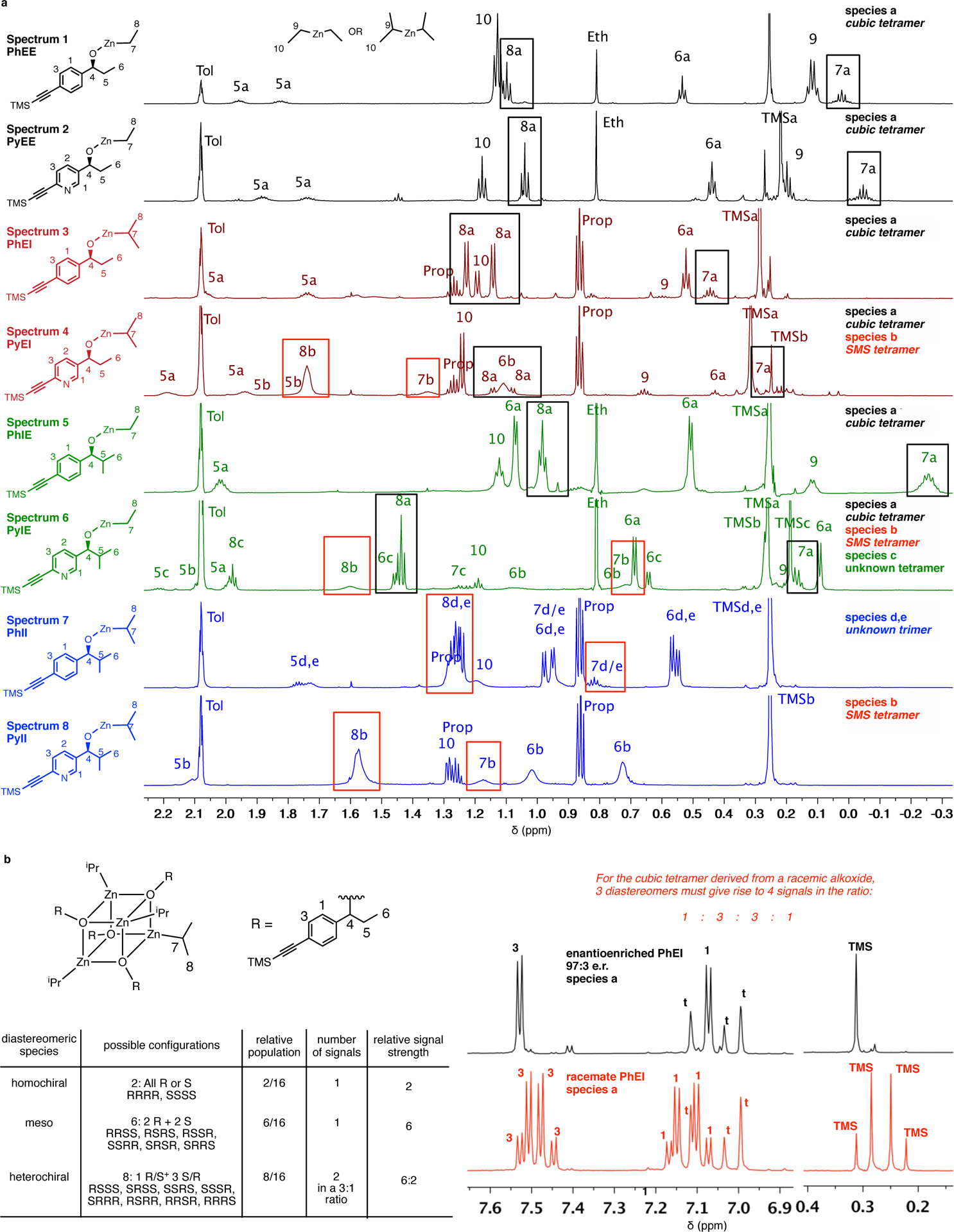
Spectroscopic characterization of zinc alkoxides reveals structure-dependent solution state aggregation. Partial 1H-NMR spectra (750 MHz) in toluene-d8 at 23 °C. tol/t = residual toluene, prop = propane, eth = ethane. Enantioenriched zinc alkoxides (alkoxides differing by only a nitrogen atom in the aromatic core are assigned the same color and are arranged successively) with signature chemical shifts for species a (black boxed) and species b (red boxed). Additional DOSY experiments (not shown here) and racemate analysis (see panel b) assist in characterizing aggregate species. Species a, observed for PhEE/EI/IE and PyEE/EI/IE, is a cubic tetramer and is characterized by sharp peaks and upfield chemical shifts for protons H(7) and H(8). In contrast, PyII exists exclusively as species b (the SMS tetramer) whose peaks are highly broadened, with downfield chemical shifts for protons H(7) and H(8). PyEI and PyIE exist as a mixture of species a and b. A third species, c (an uncharacterized tetrameric aggregate) is observed as a component of PyIE. PhII exists as a mixture of two uncharacterized trimers (species d, e)
