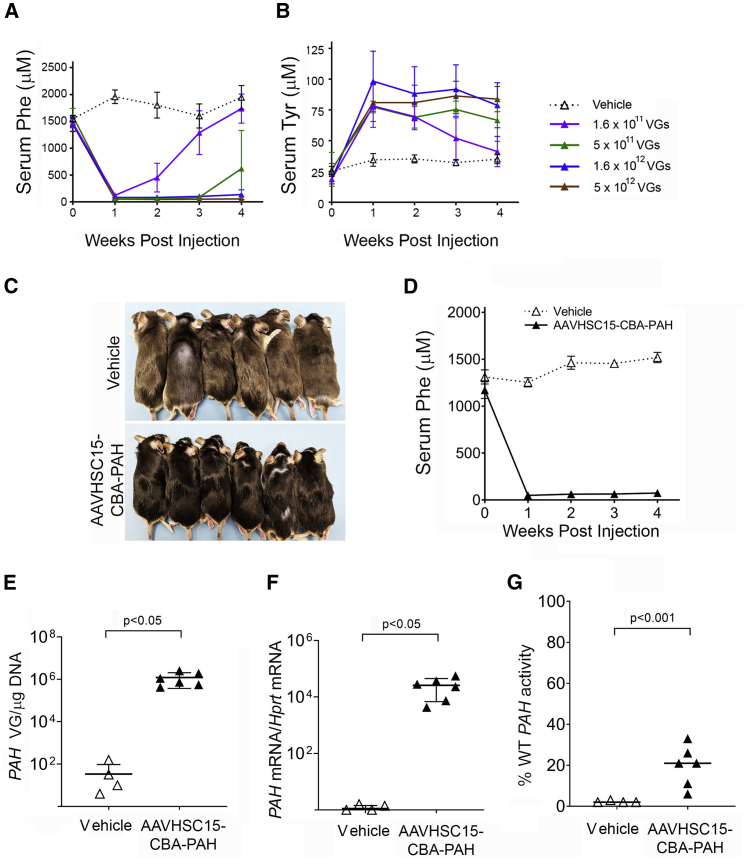
Figure 1.
Treatment of Pahenu2 Mice with AAVHSC15-CBA-PAH
(A and B) Serum Phe (A) and serum Tyr (B) levels in 7-week-old male Pahenu2 mice following intravenous administration via tail vein injection of vehicle or AAVHSC15-CBA-PAH at various doses. Serum Phe and Tyr concentrations were determined weekly for the duration of the study as described in Materials and Methods. (C) Pigmentation phenotype correction observed in male Pahenu2 mice treated with AAVHSC15-CBA-PAH at 2 × 1012 VG/mouse. (D) Serum Phe levels in 7-week-old male Pahenu2 mice following intravenous administration of vehicle (n = 4) or 2 × 1012 VG/mouse of AAVHSC15-CBA-PAH (n = 6). (E) Vector genome concentrations in liver of Pahenu2 mice treated with vehicle or 2 × 1012 VG of AAVHSC15-CBA-PAH 4 weeks after administration. Vector genomes were determined from genomic DNA extracted from liver samples as described in Materials and Methods. (F) Liver human PAH and murine mRNA Hprt levels in Pahenu2 mice treated with vehicle (n = 4) or 2 × 1012 VG of AAVHSC15-CBA-PAH (n = 6) were determined 4 weeks after administration by qRT-PCR as described in Materials and Methods. Human PAH mRNA levels were normalized to the murine housekeeping Hprt mRNA levels and expressed as human PAH mRNA/Hprt mRNA. (G) Human PAH activity in vehicle and AAVHSC15-CBA-PAH-treated Pahenu2 mice 4 weeks after administration. PAH activity was determined in liver tissue homogenates by LC-MS/MS as described in Materials and Methods. PAH activity levels were expressed as the percentage of PAH activity levels measured in liver homogenates from wild-type mice. Data in (A)–(C) are represented as mean ± SD. Statistical significance between groups in (E)–(G) was determined by the Student’s t test.