Abstract
Peripartum cardiomyopathy (PPCM) is characterized by development of left ventricular systolic dysfunction and heart failure that occurs towards the end of pregnancy or in the postpartum period in the absence of structural heart disease. A complex interplay of pathophysiological mechanisms likely contributes to the PPCM phenotype. Mutations in the mitochondrial thioredoxin reductase gene (TXNRD2) have been identified as a cause of dilated cardiomyopathy. We report a case of a shared, inherited genetic mutation in the TXNRD2 gene in a mother with PPCM and her infant son who died of dilated cardiomyopathy.
Keywords: Peripartum cardiomyopathy, Preeclampsia, Mitochondrial thioredoxin reductase gene
Highlights
-
•
Peripartum cardiomyopathy (PPCM) is characterized by left ventricular systolic dysfunction in the last month of pregnancy or first 5 months postpartum in the absence of structural heart disease
-
•
Peripartum cardiomyopathy and preeclampsia share a genetic predisposition
-
•
Detailed family history should be obtained for patients with peripartum cardiomyopathy
-
•
Genetic counseling and genetic testing should be offered to patients with peripartum cardiomyopathy
1. Introduction
Peripartum cardiomyopathy (PPCM) is defined by development of left ventricular systolic dysfunction and heart failure in the last month of pregnancy or in the first 5 months postpartum, in the absence of structural heart disease. The pathophysiological mechanisms underlying development of PPCM are complex and poorly understood. Angiogenic imbalance due to abnormal cleavage of prolactin hormone, autoimmunity, myocarditis and inherited genetic mutations are proposed mechanisms for the development of PPCM. [1] There are reports of PPCM in families of dilated cardiomyopathy (DCM), of cases of DCM in first-degree relatives of people with PPCM and of increased prevalence of truncating variants in the TTN gene in a PPCM cohort. [2,3]
We describe a case of shared genetic mutation in the thioredoxin reductase gene in a 40-year-old woman who developed pre-eclampsia and PPCM with a reduced ejection fraction, and her male infant, who developed fatal DCM. The genetic mutation was reported as a variant of unknown significance (VUS) in the thioredoxin reductase 2 gene [TXNRD2 Exon 12c.1054C > A(pPro352Thr)]. This case provides initial supporting evidence that this VUS may indeed be pathogenic.
2. Case Report
A 40-year-old African American woman, G2P0010, at 36 weeks 5 days of gestation with a history of spontaneous abortion at 6 weeks was admitted to the high-risk obstetrics service for preeclampsia after an outpatient evaluation revealed worsening hypertension and proteinuria. In the absence of severe features of preeclampsia, she was managed expectantly until 37 weeks 0 days of gestation, at which time she underwent induction of labor. Her cervical exam progressed to 4 cm dilated and 75% effaced, but despite adequate oxytocin, labor did not progress and a cesarean section was performed for a failed induction of labor. The low transverse cesarean section was performed without difficulty with an estimated blood loss of 800 ml. A well-appearing male infant was born (see details below).
Postoperatively, magnesium sulfate was administered for seizure prophylaxis given the patient's diagnosis of preeclampsia. On postoperative day 1, the patient reported shortness of breath and edema. Her heart rate was 70–80 beats per minute, blood pressure 120–130 / 70–80, and oxygen saturation was 100% on room air. Physician exam demonstrated bibasilar crackles. The patient's pulmonary edema was attributed to fluid overload in the setting of preeclampsia. Magnesium sulfate was discontinued, and furosemide 20 mg was administered intravenously. She was discharged to home on postoperative day 3.
On postoperative day 4, the patient returned to the hospital reporting difficulty breathing. She was hypertensive (187/97 mmHg) and a chest x-ray showed pulmonary edema. Cardiology was consulted for possible peripartum cardiomyopathy. A 2D echocardiogram after initiation of treatment showed mildly depressed left ventricular systolic function with left ventricular ejection fraction (LVEF) of 45% with moderate mitral regurgitation. Laboratory investigations showed an elevated brain natriuretic peptide level of 807 pg/ml (0–100 pg/ml) and troponin I level of 0.170 ng/mL (0.0–0.039 ng/ml).
She clinically improved with diuresis and medical management with captopril and carvedilol. Her left ventricular systolic function had normalized at 5-month follow-up. At 18-month follow-up, she had normal left ventricular systolic function but with elevated right- and left-sided filling pressures by echocardiogram. Brain-natriuretic peptide level was elevated at 466 pg/ml (0-100 pg/ml). The patient was asymptomatic and her functional capacity was compatible with NYHA class I.
The newborn male had 1- and 5-min Apgar scores of 8 and 9, respectively, and weighed 2.62 kg. He received phototherapy for hyperbilirubinemia and was discharged from the nursery two days after birth. A month later, he was diagnosed with dilated cardiomyopathy and underwent venoarterial ECMO (VA-ECMO) placement for cardiogenic shock. VA-ECMO was decannulated and he underwent implantation of biventricular mechanical circulatory support with oxygenator. Left ventricular biopsy at the time of mechanical circulatory support showed fibrosis and myocyte hypertrophy. He was listed for heart transplantation. At five months of age, the infant died of multiorgan failure. Autopsy was performed. Detailed histopathological examination of the heart revealed thin fibrotic walls and severe myocyte loss in right-sided heart chambers. There was extensive myocyte loss in the left ventricle with thick fibrotic epicardium and absent mid myocardium.
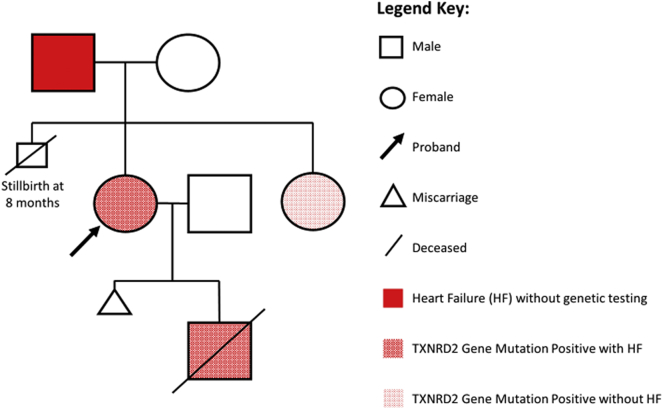
Genetic testing of the infant using a comprehensive cardiomyopathy panel that includes 69 genes for deletions, duplications or sequence changes was performed by Invitae. Testing identified a variant of uncertain significance (VUS) in the thioredoxin reductase 2 gene [TXNRD2 Exon 12c.1054C > A(pPro352Thr)]. Genetic testing was then performed on the mother and revealed the identical VUS in the thioredoxin reductase 2 gene. Genetic testing of proband's sister revealed the same genetic mutation. The sister had no prior pregnancies or history of heart failure. A detailed family history obtained by the genetic counselor revealed that the patient's father had a history of late onset congestive heart failure. The proband's father was offered genetic testing but declined. The proband's mother tested negative for gene mutation (Fig. 1).
Fig. 1.
Genetic pedigree of patient with PPCM.
PPCM: Peripartum cardiomyopathy; TXNRD2: Thioredoxin reductase; HF: Heart Failure.
3. Discussion
Peripartum cardiomyopathy is a rare syndrome with complex pathophysiological mechanisms. The proposed mechanisms include abnormal cleavage of prolactin hormone due to oxidative stress, elevated soluble fms-like tyrosine-like kinase creating an antiangiogenic environment, and hemodynamic stress of pregnancy in a susceptible individual and genetic mutations [1]. Studies have investigated the prevalence of PPCM in the dilated cardiomyopathy (DCM) database and identified genetic mutations in selected dilated cardiomyopathy genes that could be pathogenic. [2,4] This case provides initial evidence that this mutation may predispose patients to dilated cardiomyopathy.
Whole-genome sequencing by next-generation sequencing (NGS) has revolutionized this field of medicine to allow better understanding of inherited conditions and development of therapeutic targets. The Multicenter Intervention in Myocarditis and Acute Cardiomyopathy (IMAC)-2 and Intervention in Myocarditis and Acute Cardiomyopathy and Investigations of Pregnancy-Associated Cardiomyopathies (IPAC) Investigators recruited women with PPCM from six cohorts and tested for genetic mutations in 43 genes associated with DCM using NGS. Truncating variants in eight genes, most commonly in titin gene (TTN-10%) as seen with familial DCM, suggests genetic predisposition of PPCM [3].
The patient discussed here developed preeclampsia and peripartum cardiomyopathy with mild left ventricular systolic dysfunction with recovery but had persistent diastolic dysfunction. Her son was diagnosed with DCM a month after birth and died awaiting heart transplantation due to multiorgan failure. Genetic testing for patient and son revealed a shared VUS in the thioredoxin reductase 2 gene [TXNRD2 Exon 12c.1054C > A(pPro352Thr)]. Thioredoxin reductases (TXNRD) are selenocysteine-containing enzymes encoded by a nuclear gene and are essential for various cell functions, including DNA synthesis, decrease oxidative stress and prevent cell death [5]. The mitochondrial TXNRD2 is present in high concentrations in heart, kidney, muscle and adrenal gland. [6] The cellular redox balance is maintained by the reduced thioredoxins by the NADPH reaction utilized by TXNRD. The thioredoxin and thioredoxin reductase enzyme prevents oxidative stress by counteracting reactive oxygen species (ROS) [7]. Gene mutations in the TXNRD2 have been linked to rare cases of dilated cardiomyopathy (1.3%) [7].
Genetic testing in our patient and son revealed they were heterozygous carriers of the TNXRD2 gene mutation with substitution of the amionacid proline by threonine at codon 352. The gene mutation was reported as VUS because of the unknown significance of its effect on protein function and disease. However, the patient did develop preeclampsia, a known risk factor for PPCM, likely due to the hemodynamic stressor of pregnancy in a susceptible individual with mutation in the mitochondrial gene that encodes an enzyme responsible for reducing oxidative stress in the cardiac myocyte. Unfortunately, the son developed dilated cardiomyopathy and died due to multi-organ failure.
Animal data provides biologic plausibility associating this variant with heart failure. Horstkotte and colleagues reported that in mice, mitochondrial thioredoxin reductase provides myocyte protection. The neonatal autopsy findings of myocyte loss in the infant reported here with this genetic finding suggests mice findings may be extrapolated to humans. [8]
Despite a common genetic variant in a family with a shared clinical diagnosis, unanswered genetic questions remain. The patient's father developed congestive heart failure later in life; however, he declined genetic testing so his status is unknown. Has the patient's sister – who also has this genetic mutation – remained well because her heart has not yet been exposed to the physiologic changes of pregnancy and/or preeclampsia that predispose a susceptible female to heart failure? Is variable expressivity the cause of the fatal form of DCM in her infant son?
Additional cases are needed to better characterize the clinical impact of this VUS. Based on the current American College of Medical Genetics Guidelines for the interpretation of sequence variants, this case provides initial supporting evidence that this VUS may indeed be pathogenic [9].
Acknowledgments
Contributors
Indranee Rajapreyar drafted the manuscript.
Rachel Sinkey drafted the manuscript.
Salpy V Pamboukian reviewed and edited the manuscript.
Alan Tita reviewed and edited the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
No funding from an external source supported the publication of this case report.
Patient Consent
Informed consent was obtained from the patient for publication of this case.
Provenance and Peer Review
This case report was peer reviewed.
References
- 1.Arany Z., Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133(14):1397–1409. doi: 10.1161/CIRCULATIONAHA.115.020491. [DOI] [PubMed] [Google Scholar]
- 2.van Spaendonck-Zwarts K.Y., van Tintelen J.P., van Veldhuisen D.J. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation. 2010;121(20):2169–2175. doi: 10.1161/CIRCULATIONAHA.109.929646. [DOI] [PubMed] [Google Scholar]
- 3.Ware J.S., Li J., Mazaika E. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N. Engl. J. Med. 2016;374(3):233–241. doi: 10.1056/NEJMoa1505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales A., Painter T., Li R. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 2010;121(20):2176–2182. doi: 10.1161/CIRCULATIONAHA.109.931220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arner E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267(20):6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 6.Spyrou G., Enmark E., Miranda-Vizuete A., Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J. Biol. Chem. 1997;272(5):2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 7.Sibbing D., Pfeufer A., Perisic T. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur. Heart J. 2011;32(9):1121–1133. doi: 10.1093/eurheartj/ehq507. [DOI] [PubMed] [Google Scholar]
- 8.Horstkotte J., Perisic T., Schneider M. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation. 2011;124(25):2892–2902. doi: 10.1161/CIRCULATIONAHA.111.059253. [DOI] [PubMed] [Google Scholar]
- 9.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]