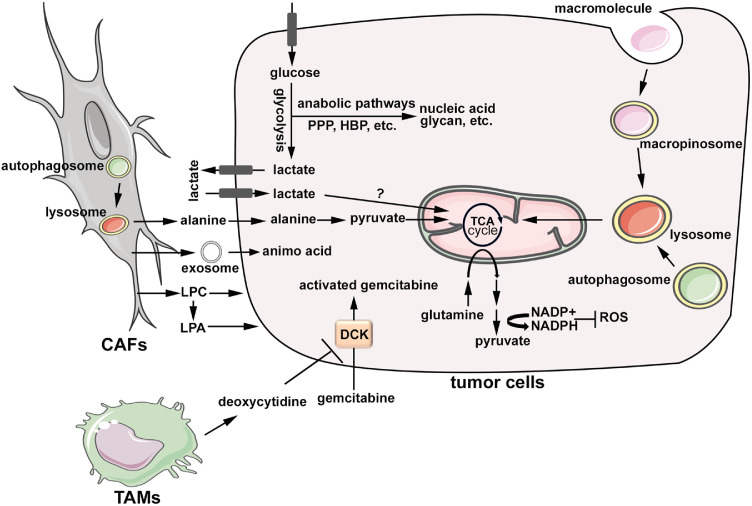
Fig. 1.
Metabolic reprogramming of neoplastic cells and crosstalk with the tumour microenvironment. Glucose uptake and glycolysis are activated in PDAC cells, which promotes the flux of glycolysis intermediates into biosynthetic pathways, including pentose phosphate pathway and hexose biosynthesis pathway, for the production of nucleic acid and glycan structures, amongst others. Tumour cells also uptake circulating lactate, which is further fed into TCA cycle through yet unknown mechanisms. Glutamine metabolism in PDAC cells is rewired to support the production of NADPH in the cytosol to maintain redox balance. PDAC cells are characterized by enhanced nutrient salvage, including the induction of macropinocytosis and autophagy, which provide substrate for energy production and anabolism in mitochondria. Autophagy in stromal fibroblasts provides alanine, which feeds into mitochondrial TCA cycle in tumour cells. Amino acids are also transferred from fibroblasts to tumour cells through exosomes. In addition, stromal fibroblasts provide phospholipids to support the proliferation of tumour cells. Tumour-associated macrophages excrete pyrimidines, in particular deoxycytidine, which competitively inhibit DCK in tumour cells to prevent gemcitabine activation and thus leading to chemoresistance. CAFs: cancer-associated fibroblasts; LPC: lysophosphatidylcholine; LPA: lysophosphatidic acid; TAMs: tumour-associated macrophages; DCK: deoxycytidine kinase; PPP: pentose phosphate pathway; HBP: hexose biosynthesis pathway; ROS: reactive oxygen species.