Graphical abstract
Keywords: Flos Lonicerae extract, Phenolic acids, Chito-oligosaccharide, Tight junction, Antiviral activity
Abstract
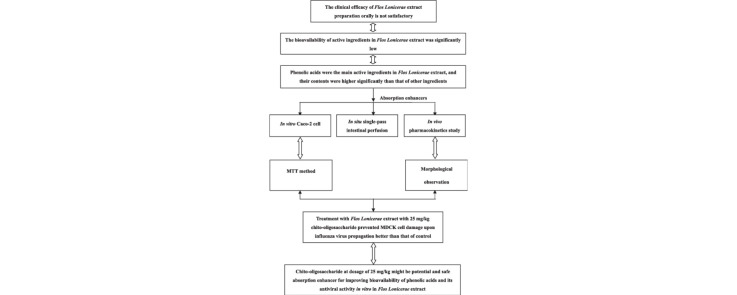
Phenolic acids, the main active ingredients in Flos Lonicerae extract possess strong antibacterial, antioxidant and antiviral effects, and their contents was higher largely than that of other ingredients such as flavones, but the absolute bioavailability orally was significantly low, which is significant low influencing clinical efficacies of its oral preparations. In the present study, in vitro Caco-2 cell, in situ single-pass intestinal perfusion and in vivo pharmacokinetics study were performed to investigate the effects of COS on the intestinal absorption of phenolic acids. The pharmacological effects such as antiviral activity improvement by COS were verified by MDCK cell damage inhibition rate after influenza virus propagation. The observations from in vitro Caco-2 cell showed that the absorption of phenolic acids in Flos Lonicerae extract could be improved by COS. Meanwhile, COS at the same low, medium and high concentrations caused a significant, concentration-dependent increase in the Papp-value for phenolic acids compared to the control group (p < 0.05), and was all safe for the Caco-2 cells. The observations from single-pass intestinal perfusion in situ model showed that the intestinal absorption of phenolic acids can be enhanced by COS. Meanwhile, the absorption enhancing effect of phenolic acids might be saturable in different intestine sites. In pharmacokinetics study, COS at dosage of 25 mg/kg improved the bioavailability of phenolic acids in Flos Lonicerae extract to the greatest extent, and was safe for gastrointestine from morphological observation. Besides, treatment with Flos Lonicerae extract with COS at dosage of 25 mg/kg prevented MDCK cell damage upon influenza virus propagation better than that of control. All findings above suggested that COS at dosage of 25 mg/kg might be safe and effective absorption enhancer for improving the bioavailability of phenolic acids and the antiviral activity in vitro in Flos Lonicerae extract.
Introduction
Flos Lonicerae, a flower of Lonicera japonica that possessed antibacterial, anti-inflammatory, antiviral, antiendotoxin, blood fat reducing, antipyretic, etc., has been widely used in traditional Chinese medicine to treat exopathogenic wind-heat, epidemic febrile diseases, sores, carbuncles, furuncles and some infection diseases (Wang 2008), and it has also been employed extensively to prevent and treat some serious viral diseases of human and veterinary, such as SASR coronavirus, H1N1 (Swine) flu virus, and being called the “bouvardin” (Jiao 2009).
Flos Lonicerae extract that was extracted from Lonicera japonica has been widely used in Chinese medicine preparations (Shang et al. 2011), such as Jin Yin Hua San, Jin Yin Hua oral liquid, Shuang-Huang-Lian freeze-dried powders of injection, Shuang-Huang-Lian oral liquid, Yin-Qiao capsule and Yin-Qiao-Jie-Du tablets, etc., which are extensively used for treating acute upper respiratory tract infection caused by virus or bacterial infection in clinical practice. And oral preparations of Flos Lonicerae extract are usually more accepted for patients than injections owing to their elimination of pain and discomfort, and lower costs to produce oral formulations, but their clinical effect was unsatisfactory compared with that of injections, which becomes one of the most limited points in the development of Chinese medicine preparations. The presumptions that whether the low bioavailability of oral Flos Lonicerae extract resulted in the poor efficacy or whether efficacy could be improved as the absorption of active ingredients in Flos Lonicerae extract was enhanced are all yet to be investigated.
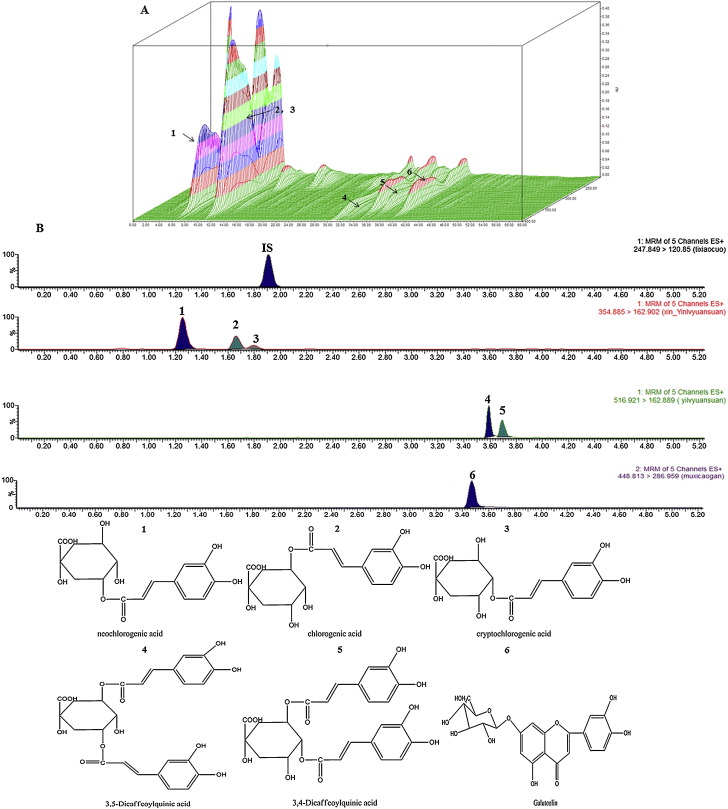
As shown in Fig. 2 and Table 1 , we found that only phenolic acids and flavones, not saponins were found in the Flos Lonicerae extract. According to the HPLC figureprint profile shown in Fig. 1(A) and UPLC–MS/MS shown in Fig. 1(B), we found that the contents of phenolic acids in Flos Lonicerae extract such as neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid or 3,4-dicaffeoylquinic acid were about 16.1, 16.9, 16.6, 18.9 and 37.8 mg/ml, respectively, higher largely than that of other ingredients, but their absolute bioavailability orally was significantly low (unpublished). Besides, the content of flavones such as galuteolin was 0.335 mg/ml, lower than that of phenolic acids such as isochlorogenic, and we also found that about 100% of the apically loaded hyperin, galuteolin, rutin were retained on the apical side from Caco-2 cell transport (unpublished). Conceptually, all compounds in the pharmaceutical product that are bioavailable in the systemic circulation can be considered as relevant active markers for pharmacological effects. Therefore, the properties above of main ingredients in Flos Lonicerae extract led us to postulate that low bioavailability of phenolic acids in Flos Lonicerae extract might result in low efficacy of clinical therapy.
Fig. 2.
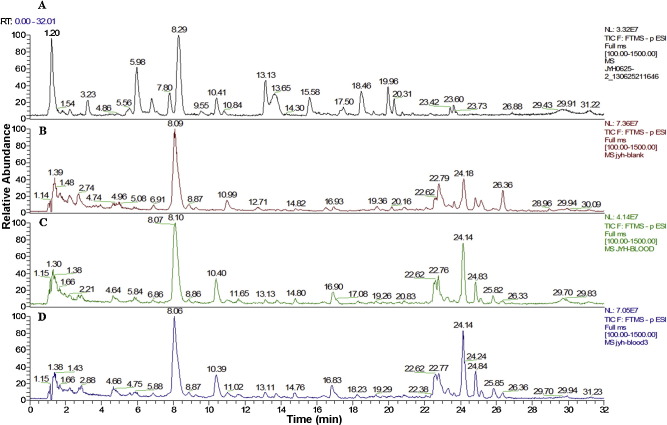
The preparation methods including solid-phase extraction (SPE), methanol precipitation, acetonitrile precipitation, liquid–liquid extraction with ethyl acetate and liquid–liquid extraction with the mixture of ethyl acetate and methanol were all tried. As a result, the acetonitrile precipitation was selected considering the number of chromatography peaks and the interference from the co-eluted endogenous matrix for samples from the hepatic portal vein. (A: Flos Lonicerae extract, B: controlled rat plasma, C: rat plasma after oral administration of Flos Lonicerae extract, D: rat plasma after oral administration of Flos Lonicerae extract with COS).
Table 1.
The preliminary study on MS and MS/MS data of the identified components in rat plasma after oral administration of Flos Lonicerae extracts in negative mode.
| Peak no. | Components | Chemical profiles in vitro | Chemical profiles in rat plasma |
ESI−, [M−H]− | Source | |
|---|---|---|---|---|---|---|
| Flos Lonicerae extracts | Flos Lonicerae extracts | Flos Lonicerae extracts + COS | MS | |||
| 1 | Caffeic acid | + | + | + | 179.03389 | Phenolic acids |
| 2 | Quinic acid | + | + | + | 191.05501 | Phenolic acids |
| 3 | Neochlorogenic acid | + | + | + | 353.08671 | Phenolic acids |
| 4 | Chlorogenic acid | + | + | + | 353.08671 | Phenolic acids |
| 5 | Cryptochlorogenic a cid | + | + | + | 353.08671 | Phenolic acids |
| 6 | 3,5-Dicaffeoylquinic acid | + | + | + | 515.11840 | Phenolic acids |
| 7 | 3,4-Dicaffeoylquinic acid | + | + | + | 515.11840 | Phenolic acids |
| 8 | Luteolin | + | + | + | 285.03936 | Flavones |
| 9 | Galuteolin | + | − | − | 447.09219 | Flavones |
| 10 | Luteolin-7-o-β-d-glucopyranoside | + | − | − | 447.09219 | Flavones |
| 11 | Luteolin-7-o--β-d-galactoside | + | − | − | 447.09219 | Flavones |
| 12 | Hyperoside | + | − | − | 463.08710 | Flavones |
| 13 | Apigenin | + | + | + | 269.04445 | Flavones |
| 14 | Quercetin | + | + | + | 301.03428 | Flavones |
| 15 | Naringenin | + | + | + | 271.06010 | Flavones |
| 16 | Rutin | + | − | − | 609.14501 | Flavones |
| 17 | Diosmetin-7-o-β-d-glucopyranoside | + | − | − | 461.10784 | Flavones |
| 18 | Genistein | + | + | + | 269.04445 | Flavones |
| 19 | Genistein-5-o-β-d-glucopyranoside | + | − | − | 431.09727 | Flavones |
| 20 | Chrysoeriol-7-o-β-d-glucopyranoside | + | − | − | 461.10784 | Flavones |
| 21 | Isorhamnetin-3-o-β-d-glucopyranoside | + | − | − | 477.10275 | Flavones |
| 22 | Kaempferol-3-o-β-d-glucopyranoside | + | − | − | 447.09219 | Flavones |
| 23 | 5,7,4′-Trihydroxyflavanone-7-o--β-d-glucopyranoside | + | − | − | 433.11292 | Flavones |
| 24 | Cosmosiin | + | − | − | 431.09727 | Flavones |
| 25 | Apigenin-7-o-neohesperidoside | + | − | − | 577.15518 | Flavones |
| 26 | Apigenin-7-o-gentibioside | + | − | − | 593.15010 | Flavones |
| 27 | Apigenin-7-o-sophoroside | + | − | − | 593.15010 | Flavones |
| 28 | Secosyloganin | + | − | − | 403.12349 | Iridoids |
| 29 | Luteolin of methylation | − | + | + | 461.10784 | Metabolites |
| 30 | Luteolin-o-glucuronide | − | + | + | 461.07145 | Metabolites |
| 31 | Apigenin of methylation | − | + | + | 283.06010 | Metabolites |
| 32 | Apigenin-o-glucuronide | − | + | + | 446.08436 | Metabolites |
| 33 | Quercetin of methylation | − | + | + | 315.04993 | Metabolites |
| 34 | Quercetin-o-glucuronide | − | + | + | 477.06637 | Metabolites |
| 35 | Genistein of methylation | − | + | + | 283.06010 | Metabolites |
| 36 | Genistein-o-glucuronide | − | + | + | 445.07654 | Metabolites |
| 37 | Naringenin of methylation | − | + | + | 285.07575 | Metabolites |
| 38 | Naringenin-o-glucuronide | − | + | + | 447.09219 | Metabolites |
| 39 | Cosmosiin of methylation | − | + | + | 371.11253 | Metabolites |
| 40 | Cosmosiin of glucuronide | − | + | + | 533.12897 | Metabolites |
Fig. 1.
(A) 3D HPLC-profile of MeOH (50%) soluble portion of Flos Lonicerae extract; the MeOH soluble portion of Flos Lonicerae extract was filtered through a 0.45 μm membrane filter and the resulting filtrate was subjected for HPLC analysis. The analyses were performed using a Waters 2695 Alliance HPLC system (Waters Corp., Milford, MA, USA), consisting of a quaternary pump solvent management system, an on-line degasser, and an autosampler. The raw data were detected by 2998 PDA, acquired, and processed with Empower™ software. A Hypersil ODS C18 column (250 × 4.6 mm, 5 μm) (Thermo Scientific, Waltham, MA, USA) was applied for all analyses. The mobile phase was composed of A (acetonitrile) and solvent B (0.4% aqueous phosphoric acid, V/V) with a linear gradient elution: 0–20 min, 10–14% A; 20–50 min, 14–30% A; 50–55 min, 30–10% A; 55–60 min, 10% A. The mobile phase flow rate was 1 ml min−1, the column temperature was controlled at 30 °C and sample injection volume was 10 μl. (B) UPLC–MS/MS profile of Flos Lonicerae extract to determination phenolic acids simultaneous (unpublished).
In our previous study, we found that phenolic acids instead of flavones in Flos Lonicerae extract were survived good positive correlation between dose and effects of antibacterial, antioxidant and antiviral via pharmacokinetic/pharmacodynamic (PK/PD) model combined with PLS (partial least squares), and the antibacterial, antioxidant and antiviral activities were improved as the contents of phenolic acids in Flos Lonicerae extract were enhanced, and showing obvious correlation between dose of phenolic acids and pharmacological effects. Besides, it was found that those phenolic acids especially isochrogenic acids in Flos Lonicerae extract were the main bioactive components for antioxidant, antiviral and antibacterial effect by “Spectrum-activity relationship” and “Knock-out/Knock-in” methods (Wang, 2010, Zhang, 2011). Therefore, how to improve the bioavailabilities of phenolic aicds would be related to pharmacological efficacy improvement of Flos Lonicerae extract directly.
According to our previous study, the intestinal absorption mechanism of phenolic acids was passive diffusion, and involved paracellular route transport mainly governed by the tight junctions (TJs) (Konishi and Kobayashi 2004), and the modulation of the TJs by absorption enhancers would enhance the paracellular drug transport (Salama et al. 2006).
It was reported that absorption enhancers including surfactants, bile salts and chelating agents, etc. was one of the most promising methods to improve the bioavailability of poorly absorbable drugs orally (Uchiyama et al. 1999). Recently, it was demonstrated that nitric oxide (NO) donors and polyamines were also effective for improving the intestinal absorption of poorly absorbable drugs (Fetih et al. 2005). However, some absorption enhancers can cause damage and irritate the intestinal mucosal membranes. This is a limiting factor for their clinical use. Indeed, there existed almost linear relationship between the effectiveness of various absorption enhancers and their membrane toxicity reported by Yamamoto et al. (1996). Therefore, the absorption enhancers based on tight junctions with high effectiveness and low mucosal toxicity need to be investigated.
Chitosan, a natural polymer obtained by alkaline deacetylation of chitin, is non-toxic, biocompatible, and biodegradable. The ability of chitosan to enhance gastrointestinal (GI) drug absorption has been of special interest (Gao et al. 2008). Illum et al. (1994) proposed that the mechanism of absorption enhancement was a combination of mucoadhesion and a loosening effect on the tension of the tight junctions through ionic interactions with negatively charged groups of glycocalix. However, the polymer was only soluble in an acidic environment in which the pH was less or if the order of the pK a value of chitosan (5.5–6.5), which restricted its application to the absorption enhancer seriously. For example, the reduction of TEER of Caco-2 cell monolayers was found after the apical incubation with chitosan hydrochloride and chitosan glutamate at a pH of 6.20, but no decrease of TEER, which is a good measurement of the tightness of the junctions between the cells, was observed at a pH of 7.40. At this pH, both chitosan salts (hydrochloride, glutamate) did not form clear solutions. In agreement with the results of the TEER experiments, no increase in the transport of the hydrophilic model compound [14C]-mannitol was found at a pH of 7.40 after incubation with these chitosan salts (Jonker et al. 2002). Therefore, the potential use of chitosans, as absorption enhancer in the more basic environments of intestine, was limited.
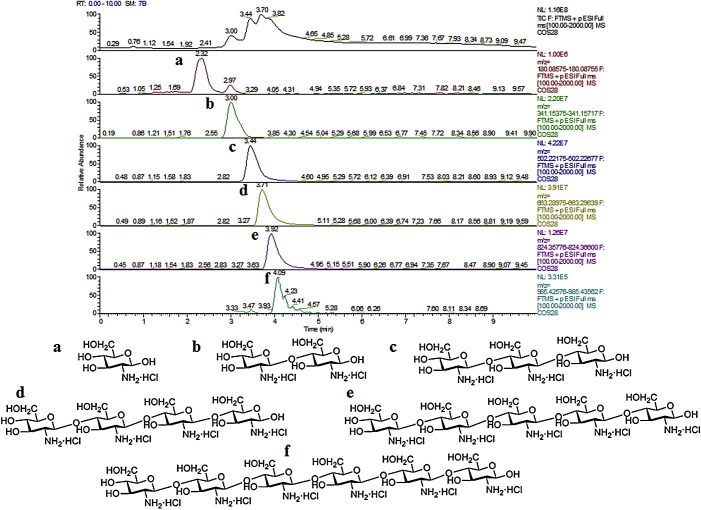
Chitosan derivatives have been suggested as promising excipients for absorption enhancement of GI drug in cases in which additional physicochemical properties in the polymer structure were desirable. Chito-oligosaccharide (COS) shown in Fig. 3, a new type of chitosan molecular, had remarkable solubility in water at physiological pH due to its low molecular weight, and we found that the contents of d-glucosamine, chitosan dimer, chitosan trimer, chitosan tetramer, chitosan pentamer and chitosan hexamer were 0.42%, 9.19%, 18.78%, 0.69%, 14.48% and 2.56% of totally, respectively. Gao et al. (2008) reported the effect of chitosan oligomers on the intestinal absorption of hydrophilic macromolecular drugs, such as fluorescein isothiocyanate-labeled dextrans (FD4), and found that the bioavailability of FD4 could be all increased by about 2 times by chitosan dimer (0.5%, W/V), 1.5 times by chitosan trimer (0.5%, W/V) and 2.4 times by chitosan hexamer (0.5%, W/V) from jejunum. Besides, a moderate increase in the colonic absorption of FD4 was observed in the presence of chitosan pentamer (0.5%, W/V) and dimer (0.5%, W/V) from in situ loop method in rats.
Fig. 3.
The production and composition of chito-oligosacharide. The analyses were performed using ultra-high performance liquid chromatography with Thermo Scientific Orbitrap mass spectrometry systems (UHPLC–Orbitrap MS). And all mass spectra were acquired in the positive ion mode, within the mass range 100–2000 m/z. Separation was achieved using Welch ultimate XB-NH2 (100 × 2.1 mm, 3 μm). The injection volume used was 5 μl. The mobile phase was composed of A (acetonitrile) and solvent B (0.3% aqueous ammonia, V/V) with a linear gradient elution: 0–2 min, 75–40% A; 2–5 min, 40–40% A; 5–8 min, 40–75% A, and held for 2 min, at a flow rate of 400 μl/min, resulting chromatographic full-width-at-half-maximum (FWHM) of 3–5 s. Analytical data were collected at resolution from 30,000 and scanning rate 1–12 scans/s.
Therefore, the current study arms to demonstrate the effect of COS on the intestinal absorption of phenolic acids in Flos Lonicerae extract using in vitro, in situ and in vivo models.
Materials
Flos Lonicerae extract was purchased from Jiangyin Tianjiang Pharmaceutics Co, Ltd. To assure the homogeneity of the formulation and to prepare consistent hatched, the HPLC figureprint profile Fig. 1(A) of the Flos Lonicerae extract was analyzed, and the chromatographic analysis was carried out under the previous study (Chinese Pharmacopoeia Commission, 2010, Chen et al., 2007, Chen, 2007, Wagner et al., 2011). A reliable UHPLC–Orbitrap MS system was established for detecting the prototype compounds in Flos Lonicerae extract and dosed plasma with or without COS, respectively (Fig. 2 ). All voucher specimens were deposited in our laboratory for future reference. Chlorogenic acid, galuteolin and tinidazole (using as internal standard, IS) were purchased from National Institute for the Control of Pharmaceutical and Biological Products. Neochlorogenic acid, cryptochlorogenic acid, 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid (98% pure) was purchased from Sichuan Weikeqi Bio-tech Co., Ltd. COS (Fig. 3 ) was all purchased from Qingdao Honghai Bio-tech Co., Ltd. Heparin Sodium Injection was purchased from Changzhou Qianhong Bio-pharma Co., Ltd. Methanol and acetonitrile (HPLC grade) were purchased from Merck (Merck, Germany), and water was purified by a Milli-Q water purification system (Millipore, Bedford, MA, USA). All other chemicals and reagents were of analytical grade.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), 0.05% trypsin-EDTA, penicillin-streptomycin and non-essential amino acids were obtained from GibcoBRI, Life and Technologies, USA. Collagen type I, sodium pyruvate, MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide) and trypsin_TPCK (Tosylamide Phenylethyl Chloromethyl Keton-treated Trypsin) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). HBSS (Hank's balanced salt solution) and PBS (Phosphate Buffered Saline) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Culture cell inserts for 6 well plates (CCI, 137435) were purchase from Nalge Nunc International. (Roskilde, Denmark).
The human colorectal cancer cell lines (Caco-2, HCT116) were bought from cell bank (Chinese Academy of Sciences). Madin-Darby canine kidney cell lines (MDCK cell, KG067) were purchased from Keygen biotech Co., Ltd. The influenza virus strain, A/PR8/34 (H1N1) was purchased from Chinese Academy of Preventive Medicine.
Male Sprague-Dawley (SD) rats (∼250 g) were supplied by the Experimental Animal Center of Nanjing University of Chinese Medicine (Certificate No.SCXK2008-0033). The experimental procedures were in compliance with the animal ethics committee of the Nanjing University of Chinese Medicine.
Methods
Effect of COS on the intestinal absorption of chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid in Flos Lonicerae
In vitro Caco-2 cell monolayer model
Caco-2 cells were cultured in high glucose DMEM with 10% fetal bovine serum, 1% nonessential amino acids. Cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C. After reaching 80% confluens, Caco-2 cells were harvested with 0.05% trypsin-EDTA solution and seeded on top of CC inserts in 6-well plates, which has a surface area of 4.2 cm2, at a density of 1.0 × 105 cells/cm2. The protocols for cell culture in Transwell inserts were similar to those described previously (Zhou et al., 2012a, Zhou et al., 2012b).
Cell culture experiments were described previously (Zhou et al., 2012a, Zhou et al., 2012b). Briefly, after culture medium was aspirated, the cell monolayers were washed three times with blank HBSS. The transepithelial electrical resistance (TEER) values of cell monolayers were measured, which were more than 250 Ω cm2. The monolayers were incubated with the blank HBSS for 1 h with 37 °C. Thereafter the incubation medium was aspirated. Afterwards, a solution containing the compound was loaded onto the apical side. Donor samples (400 μl) (Apical side) and receiver samples (400 μl) (Basolateral side) were taken at different times (typically 1 h), followed by the addition of 400 μl drug donor solution to the donor side (AP) and 400 μl of blank buffer to the receiver side (BL). The samples were taken at 0, 1, 2, 3 and 4 h after incubation. At the end of the transport experiment, integrity of the monolayer was monitored by TEER value.
Rat in situ single pass intestinal perfusion study
Rat in situ single pass intestinal perfusion model was set at previously described by us (Zhou et al., 2012a, Zhou et al., 2012b, Du et al., 2009). Briefly, SD rats (body weight: 250–300 g) were fasted overnight with free access to water. The rats were anesthetized with 20% urethane solution (6 mg/kg). A midline abdominal incision was made and the small intestine was exposed. The bile duct was ligated in order to avoid bile secretion into the perfusate. For the regional absorption of drugs, three intestinal sections were isolated and cannulated (all were 10 cm long): duodenum, jejunum and ileum. Each segment was rinsed with normal saline at 37 °C for 20 min until the washing appeared clear. After that, the perfusion solution of drugs as solvent was connected to the each segment and perfusing through each part of the three intestine sections. At the beginning of 30 min, the circulation rate was 0.2 ml min−1 controlled by a peristaltic pump to pre-balance, then, perfusate samples were collected. Solutions containing 20 μM for chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid, 15 μM for 3,5-dicaffeoylquinic acid and 30 μM for 3,4-dicaffeoylquinic acid were perfused through the intestinal lumen to investigate the permeabilities of the two studied compounds in the rats in situ intestinal perfusion model. In addition, 20 μM for chlorogenic acid, neochlorogenic acid and cryptochlorogenic acid, 15 μM for 3,5-dicaffeoylquinic acid and 30 μM for 3,4-dicaffeoylquinic acid with the addition of COS were also perfused through the intestinal lumen to investigate the effect of chitosan derivatives on the permeabilities of the two studied compounds. The perfusate samples were collected at 30–60, 60–90, 90–120 and 120–150 min, and stored at −80 °C refrigerator until analysis.
Rat in vivo pharmacokinetics study
Product Flos Lonicerae extracts was dissolved in saline with or without COS to give the concentration of 60% (v/v) immediately prior to drug administration. The contents of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid and COS were determined to be 16.1, 16.9, 16.6, 18.9, 37.8 and 6.25 mg/ml of extracts, respectively.
Male SD rats (∼250 g) were kept in an environmentally controlled breeding room (temperature: 20 ± 2 °C, relative humidity: 60 ± 5%) for 1 week. The animals were fasted for 12 h prior to drug administration of Product Flos Lonicerae extracts with or without COS prepared with a dose of 10 ml/kg. After dosing for 0, 5, 10, 15, 20, 30, 40, 55, 70, 100, 160, 250, 480 min, blood was collected from the pre-intubated catheter and put into tubes with heparin sodium injection (10 μl) and ascorbic acid (2 μg) at predetermined time points. Subsequently, plasma was prepared by centrifugation at 1816 × g for 7 min and stored at −80 °C for further analysis.
Sample preparations and analysis
Sample treatment and UPLC–MS/MS analysis for samples collected from in vitro, in situ and in vivo models, respectively were described previously (unpublished).
Effect of Flos Lonicerae with or without COS on influenza virus
MDCK cells were grown in DMEM as described previously (Mehrbod et al. 2009), supplemented with 10% FBS and 1% Pen/Strep at 37 °C in a humidified incubator. The media was changed two to three times per week. The influenza virus was propagated in MDCK cells in the presence of 1 μg/ml of Trypsin_TPCK to create the working stock. During antiviral evaluations, media supplemented with FBS was sucked out and the cell washed with PBS and then it was treated as needed. The media supplemented with Trypsin_TPCK was added.
Serum after administration orally into Flos Lonicerae extracts with or without COS at the dosages of 25 mg/kg was added to the MDCK cells after the propagation with influenza virus. The cells were incubated at 37 °C for 48 h before viability testing by measuring the conversion of MTT as described by us (Zhou et al., 2012a).
Calculation
For Caco-2 monolayer model, the apparent permeability coefficient (P app) was calculated as P app = [(dQ/dt)]/[A × C], dQ/dt (μg/S) was the flux rate, A was the effective surface area of the cell monolayer (4.2 cm2), and C 0 (μg/ml) was the initial drug concentration in the donor chamber. For rat single-pass intestinal perfusion in situ model, the concentration of perfusion fluid was calculated as C out(corrected) = C out PR in/PR out and the effective permeability coefficient (P eff) was calculated as P eff = Qln[C in/C out(corrected)]/2πrL. C out(corrected) was effluent drug concentration with correction; C out was effluent drug concentration without correction; C in was influent drug concentration; PR in was influent phenol red concentration; PR out were effluent phenol red concentration; Q was perfusate flow rate; r was radius of intestinal segment and l was intestinal segment length. Inhibition rate = [OD(drug) − OD(model)]/[OD(control) − OD(model)].
Pharmacokinetic analysis
The peak concentrations (C max) and the time to reach the peak concentrations (T max) were determined directly from the plasma concentration–time profiles. The area under the curve (AUC) was calculated by the trapezoidal method from time zero to the final sampling. The absorption enhancement ratios of drugs with or without enhancers were calculated as Absorption enhancement ratio = AUC with enhancer/AUC control(without enhancer).
Statistical analysis
Statistical significance in the P app, P eff values, pharmacokinetic parameters and inhibition rate index obtained from various treatment groups was estimated by the analysis of variance (Student t-test) or one-way ANOVA. A p value of less than 0.05 was considered to be significantly different. All data were expressed as mean ± SD.
Results
Effect of COS on the Papp-value of phenolic acids in the apical-to-basolateral (AP-BL) direction in vitro Caco-2 monolayer model
As shown in Fig. 4 , COS at the low, medium and high concentrations caused a significant, concentration-dependent increase in the P app-value for neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid and 3,4-dicaffeoylquinic acid compared to the control group (p < 0.05). The highest P app-value for neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid and 3,4-dicaffeoylquinic acid was increased to 5700% (9.11 ± 0.43) × 10−6 cm/s, 9800% (18.97 ± 3.35) × 10−6 cm/s, 12,800% (14.10 ± 1.4) × 10−6 cm/s, 3900% (13.90 ± 0.10) × 10−6 cm/s and 9800% (13.00 ± 0.30) × 10−6 cm/s with addition of 0.1% (w/v) of COS.
Fig. 4.
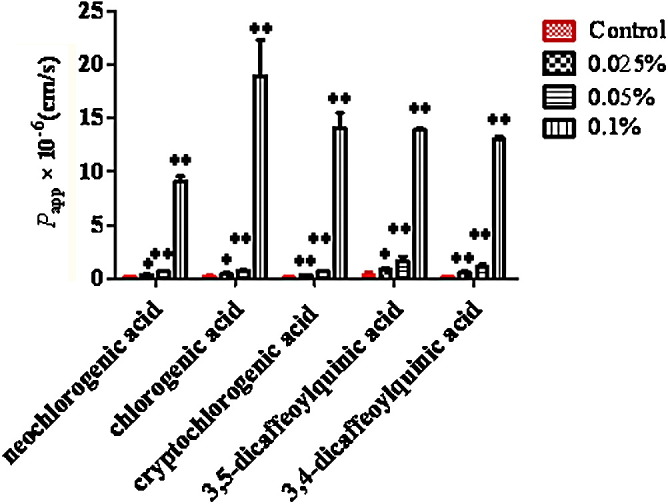
Effects of COS on absorption parameters of phenolic acids in Caco-2 cell in vitro model. Results are expressed as the mean ± S.D. *p < 0.05 and **p < 0.01 compared with the control group.
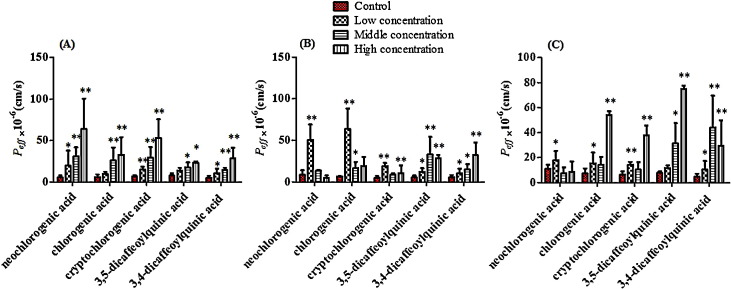
Effect of COS on the bioavailability of phenolic acids in situ single pass intestinal perfusion model
As shown in Fig. 5 , COS led to dose-dependent P eff-values of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid in duodenum and ileum, but in jejunum COS at low dose yielded maximum absorption, and the P eff-values of 3,5-dicaffeoylquinic acid in duodenum and ileum showed dose-dependent manner, but in jejunum COS at moderate concentration reached maximum absorption. Besides, COS gave P eff-values of 3,4-dicaffeoylquinic acid in a dose-dependent manner in duodenum and jejunum, but at moderate dose made 3,4-dicaffeoylquinic acid was absorbed maximally in ileum. The results indicated that the intestinal absorption of phenolic acids can be enhanced by COS. Meanwhile, the absorption enhancing effect of phenolic acids might be saturable in different intestine sites.
Fig. 5.
Effects of COS on absorption parameters of phenolic acids in rat single pass intestinal perfusion via duodenum (A), jejunum (B) and ileum (C) in situ model. Results are expressed as the mean ± S.D. *p < 0.05 and **p < 0.01 compared with the control group.
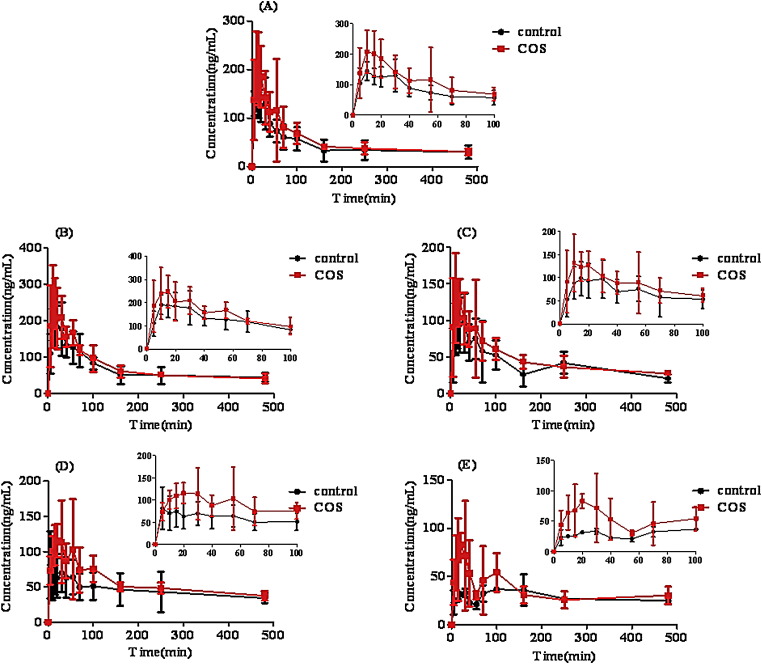
Effect of COS on the bioavailability of phenolic acids in vivo pharmacokinetics study
As shown in Fig. 6 and Table 2 , the C max of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid and 3,4-dicaffeoylquinic acid was increased to 150% (223.72 ± 80.87) ng/ml, 136% (283.73 ± 76.84) ng/ml, 164% (151.47 ± 64.79) ng/ml, 215% (137.80 ± 50.60) ng/ml and 226% (96.60 ± 41.33) ng/ml, respectively compared to the control groups. Besides, the AUC of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, 3,5-dicaffeoylquinic acid and 3,4-dicaffeoylquinic acid was increased to 187% (26,138.20 ± 7557.43) ng min/ml, 122% (39,458.00 ± 6080.64) ng min/ml, 192% (27,442.00 ± 2691.25) ng min/ml, 170% (26,936.00 ± 3834.91) ng min/ml and 177% (26,052.00 ± 2140.68) ng min/ml, respectively with the addition of COS at the dosage of 25 mg/kg, although we found that in the previous study that COS at the dosage of 25 mg/kg could result in the maximum enhancing absorption for polyphenol components like chlorogenic acid (unpublished). Therefore, these findings indicated that 25 mg/kg COS would be the promising enhancer for improving the bioavailability of phenolic acids in Flos Lonicerae extract in rats.
Fig. 6.
Plasma concentration–time profiles of phenolic acids in Flos Lonicerae extract (equivalent to 96.6 mg/kg for neochlorogenic acid, 101.4 mg/kg for chlorogenic acid, 99.6 mg.kg for cryptochlorogenic acid, 113.4 mg/kg for 3,5-dicaffeoylquinic acid and 226.8 mg/kg for 3,4-dicaffeoylquinic acid) with COS after administration to the rat gastrointestine by in vivo pharmacokinetics study. Results are expressed as the mean ± S.D. of 3–5 experiments.
Table 2.
Pharmacokinetic parameter of chlorogenic acid, neochlorogenic acid, cryptochlorogenic acid, 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid in rats after oral administration of Flos Lonicerae extracts with or without the addition of COS (mean ± S.D., n = 5).
| Cmax (ng/ml) | Tmax (min) | AUC0–480min (ng min/ml) | Enhancement ratio | ||
|---|---|---|---|---|---|
| Neochlorogenic acid | Control | 149.05 ± 33.40 | 24.00 ± 8.22 | 13,988.00 ± 5215.62 | – |
| COS | 223.72 ± 80.87 | 14 ± 4.18 | 26,138.20 ± 7557.43* | 1.87 | |
| Chlorogenic acid | Control | 236.45 ± 61.18 | 30.00 ± 0.00 | 32,430.33 ± 7650.92 | – |
| COS | 283.73 ± 76.84 | 13.75 ± 4.79 | 39,458.00 ± 6080.64* | 1.22 | |
| Cryptochlorogenic acid | Control | 134.94 ± 49.24 | 30.00 ± 10.00 | 14,315.00 ± 1097.43 | – |
| COS | 151.47 ± 64.79 | 14.00 ± 4.18 | 27,442.00 ± 2691.25* | 1.92 | |
| 3,5-Dicaffeoylquinic acid | Control | 73.30 ± 28.58 | 36.25 ± 12.50 | 16,235.00 ± 4632.96 | – |
| COS | 137.80 ± 50.60 | 22.50 ± 5.00 | 26,936.00 ± 3834.91* | 1.70 | |
| 3,4-Dicaffeoylquinic acid | Control | 65.77 ± 36.48 | 27.50 ± 5.00 | 14,714.67 ± 2595.60 | – |
| COS | 96.60 ± 41.33 | 17.50 ± 2.89 | 26,052.00 ± 2140.68* | 1.77 |
*p < 0.05 and **p < 0.01 compared with the control group.
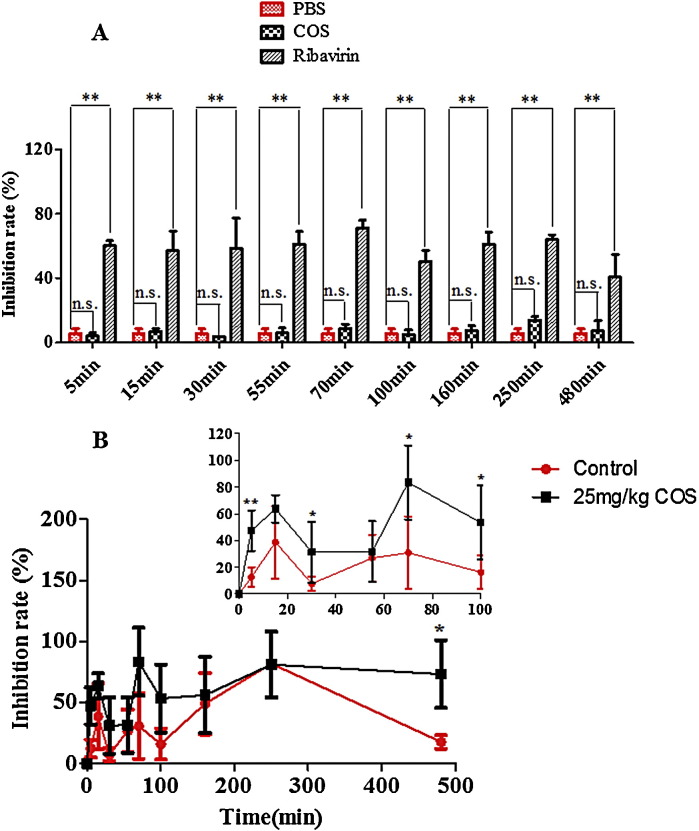
Effect of Flos Lonicerae extract with or without COS on influenza virus
As shown in Fig. 7(A), the antiviral model was built successfully, and the inhibition rate of COS group was no significant compared with that of PBS group, although there was a remarkable increase in inhibition rate value after administrating orally 20 mg/kg ribavirin as a positive control. However, the difference of antiviral activity between Flos Lonicerae extract with or without COS was significant from Fig. 7(B). The inhibition rate of Flos Lonicerae extract with COS at dosage of 25 mg/kg was higher than that of without COS, which indicated that the pharmacological effects such as antiviral effect of Flos Lonicerae extract could be significantly improved by addition of COS.
Fig. 7.
Effect of Flos Lonicerae extract with or without COS at the dosage of 25 mg/kg on influenza virus (A: inhibition rate of COS and ribavirin on influenza virus; B: inhibition rate of Flos Lonicerae extract with or without COS on influenza virus); inhibition rate was assayed with MTT 48 h later and expressed as percentage of controls (data ± S.D. n = 8). *p < 0.05, **p < 0.01 and (N.S.) no significant difference.
Discussions
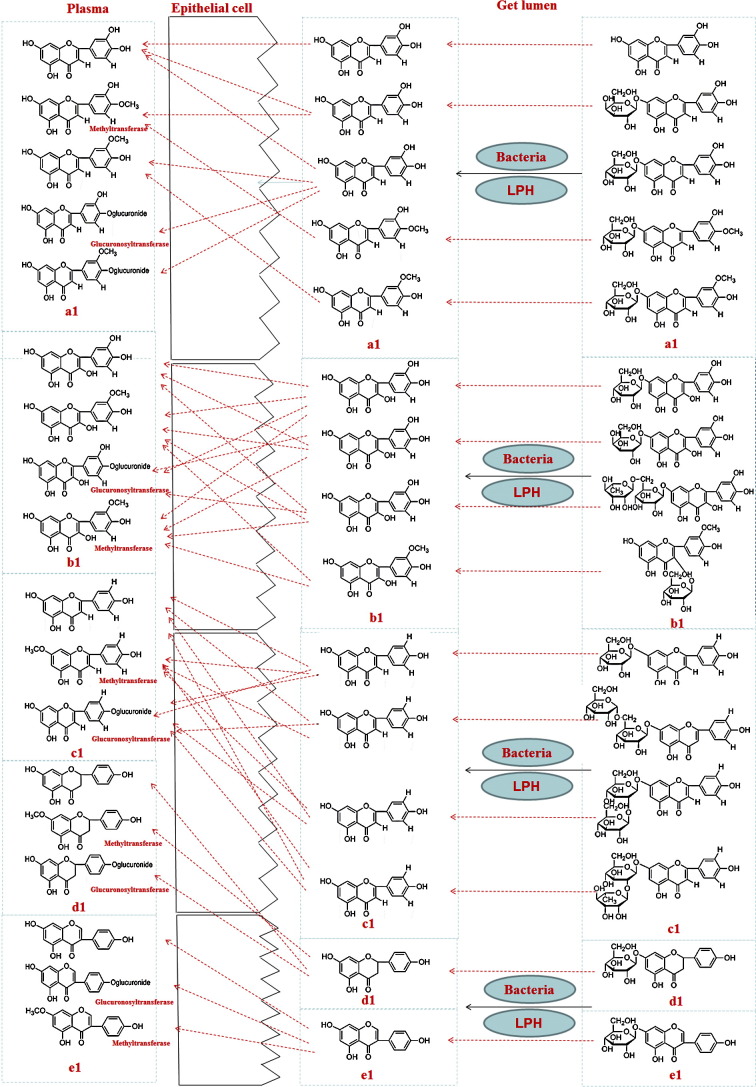
In the present study, we found from Fig. 2 and Table 1 that 24 peaks were detected in dosed plasma with or without COS but not in control plasma by comparing the chemical profiles of dosed plasma with control plasma in negative ion mode. Among these peaks, phenolic acids and flavonoids aglycone containing 12 peaks also appeared in the MS spectra of Flos Lonicerae extract, indicating that these components may be absorbed into the rat plasma in the original form. The result was consistent with the previous report that flavonoids glucoside like galuteolin, luteolin-7-o-β-d-glucopyranoside, luteolin-7-o--β-d-galactoside, hyperin, hyperoside and rutin cannot be well absorbed into Caco-2 cell, which suggested that most of flavonoids glycosides needed to be transformed into flavonoids aglycone, and then be absorbed into the plasma Fig. 8 , and the real active ingredients in flavonoids may be flavonoids aglycone, not flavonoids glycosides. However, flavonoids glycosides in vivo can not be quantitative assessed well illustrated by Fig. 8. Meanwhile, as shown in Fig. 7, treatment with Flos Lonicerae extract with COS improves MDCK cell viability better after influenza virus propagation. All evidence above supporting the hypothesis that phenolic acid can be considered as one of the important marker compounds to control the pharmacology of Flos Lonicerae extract.
Fig. 8.
Possible absorption and metabolism pathway for flavones.
We also found that the absorption enhancing effect of COS for improving the intestinal absorption of phenolic acids was affected by their concentrations, a maximal absorption enhancing effect of COS was observed at a dosage of 25 mg/kg, not higher doses. Gao et al. (2008) reported the absorption enhancing effect of chitosan hexamer for FD4 was dependent on its concentration, but its absorption enhancing effect was almost saturable up to 0.5% (w/v) from in situ loop method. Therefore, our present result is consistent with the previous report and chitosan has some optimal concentrations to show the greatest absorption enhancing effects for improving the intestinal absorption of phenolic acids in Flos Lonicerae extract, although the type of chitosan was different.
The present study also demonstrated that the absorption of phenolic acids in Flos Lonicerae extract can be not only improved greatest, but also safety in gastrointestine by COS at the dosage of 25 mg/kg. Meanwhile, Thanou et al., 2001, Thanou et al., 2007 showed the effect of low viscosity grade Mono-N-Carboxymethyl Chitosan (LMCC), High low viscosity grade N-sulfonato-N,O-carboxymethy-lchitosan (SNOCC-60) on the absorption of low molecular weight heparin (LMWH), and found that the AUC values can be increased by 7 and 18 times, respectively by intraduodenally administration. Therefore, other chitosan derivatives as absorption enhancers need to be further investigated in order to improve the pharmacological effects of Flos Lonicerae extract better.
Acknowledgements
The present study is supported financially by the National Natural Science Foundation of China (81001499), the Jiangsu Natural Science Foundation (BK2010560), “Qing Lan” Project from Jiangsu Provincial Technology Innovation Team Support Scheme, the priority Academic Program Development of Jiangsu Higher Education Institution (No.ysxk-2010) and 2012 program sponsored for scientific innovation research of college graduate in Jiangsu province (623).
References
- Chen C.Y., Qi L.W., Li H.J., Li P., Yi L., Ma H.L., Tang D. Simultaneous determination of iridoids, phenolic acids, flavonoids, and saponins in Flos Lonicerae and Flos Lonicerae Japonicae by HPLC-DAD-ELSD coupled with principal component analysis. J. Sep. Sci. 2007;30:3181–3192. doi: 10.1002/jssc.200700204. [DOI] [PubMed] [Google Scholar]
- Chen Y.H. Jilin Univ.; 2007. The research of fingerprints atlas about Lonicera japonica Thunb. (Master thesis (in Chinese)) [Google Scholar]
- Chinese Pharmacopoeia Commission . Chin. Med. Sci. Technol. Press; Beijing: 2010. The Pharmacopoeia of the People's Republic of China Version (2010) [Google Scholar]
- Du Q., Di L.Q., Shan J.J., Liu T.S., Zhang X.Z. Intestinal absorption of daphnetin by rats single pass perfusion in situ. Yao Xue Xue Bao. 2009;44:922–926. [PubMed] [Google Scholar]
- Fetih G., Habib F., Okada N., Fujita T., Attia M., Yamamoto A. Nitric oxide donors can enhance the intestinal transport and absorption of insulin and [Asu(1,7)]-eel calcitonin in rats. J. Control. Release. 2005;106:287–297. doi: 10.1016/j.jconrel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Gao Y., He L., Katsumi H., Sakane T., Fuiita T., Yamamoto A. Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int. J. Pharm. 2008;359:70–78. doi: 10.1016/j.ijpharm.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Illum L., Farraj N.F., Davis S.S. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 1994;11:1186–1189. doi: 10.1023/a:1018901302450. [DOI] [PubMed] [Google Scholar]
- Jonker C., Hamman J.H., Kotze A.F. Intestinal paracelluar permeation enhancement with quaternised chitosan: in situ and in vitro evaluation. Int. J. Pharm. 2002;238:205–213. doi: 10.1016/s0378-5173(02)00068-6. [DOI] [PubMed] [Google Scholar]
- Jiao S.G. Research and comprehensive utilization of honeysuckle. Qilu Pharm. Aff. 2009;28:487–489. (in Chinese) [Google Scholar]
- Konishi Y., Kobayashi S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2004;52:2518–2526. doi: 10.1021/jf035407c. [DOI] [PubMed] [Google Scholar]
- Mehrbod P., Motamed N., Tabatabaian M., Soleimani E.R., Amini E. In vitro antiviral effect of “Nanosilver” on influenza virus. Drug. 2009;17:88–93. [Google Scholar]
- Shang X.F., Pan H., Li M.X., Miao X.L., Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011;138:1–21. doi: 10.1016/j.jep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama N.N., Eddington N.D., Fasano A. Tight junction modulation and its relationship to drug delivery. Adv. Drug Deliv. Rev. 2006;58:15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Thanou M., Nihot M.T., Jansen M., Verhoef J.C., Junginger H.E. Mono-N-Carboxymethyl Chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J. Pharm. Sci. 2001;90:38–46. doi: 10.1002/1520-6017(200101)90:1<38::aid-jps5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Thanou M., Henderson S., Kydonieus A., Elson C. N-sulfonato-N, O-carboxymethylchitosan: a novel polymeric absorption enhancer for the oral delivery of macromolecular. J. Control. Release. 2007;117:171–178. doi: 10.1016/j.jconrel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Sugiyama T., Quan Y.S., Kotani A., Okada N., Fujita T., Muranishi S., Yamamoto A. Enhanced permeability of insulin across the rat intestinal membrane by various absorption enhancers: their intestinal mucosal toxicity and absorption-enhancing mechanism of n-lauryl-β-d-maltopyranoside. J. Pharm. Pharmacol. 1999;51:1241–1250. doi: 10.1211/0022357991776976. [DOI] [PubMed] [Google Scholar]
- Wang L.M. Agri. Univ. Henan; 2008. Studies on antiviral effect and immunopotentiating activity of Lonicera japonica Thunb. and flos lonicerae in vitro. (Master thesis (in Chinese)) [Google Scholar]
- Wang L. Chengdu Univ. Trad. Chin. Med.; 2010. Study of effective substances screening for flos lonicerae based on the “spectrum-effect” combination. (Master thesis (in Chinese)) [Google Scholar]
- Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A. Springer-Verlag; New York: 2011. Flos Lonicerae Japonicae. Chromatographic Fingerprint Analysis of Herbal Medicines; pp. 587–600. [Google Scholar]
- Yamamoto A., Uchiyama T., Nishikawa R., Fujita T., Muranishi S. Effectiveness and toxicity screening of various absorption enhancers in the rat small intestine: effects of absorption enhancers on the intestinal absorption of phenol red and the release of protein and phospholipids from the intestinal membrane. J. Pharm. Pharmacol. 1996;48:1285–1289. doi: 10.1111/j.2042-7158.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Zhang T.T. Chengdu Univ. Trad. Chin. Med.; 2011. Novel patterns of efficient components recognition and quality control for flos lonicerae japonicae based on constituent knock-out/knock-in. (Master thesis (in Chinese)) [Google Scholar]
- Zhou W., Qin K.M., Shan J.J., Ju W.Z., Liu S.J., Cai B.C., Di L.Q. Improvement of intestinal absorption of forsythoside A in weeping forsythia extract by various absorption enhancers based on tight junctions. Phytomedicine. 2012;20:47–58. doi: 10.1016/j.phymed.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Zhou W., Di L.Q., Wang J., Shan J.J., Liu S.J., Ju W.Z., Cai B.C. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol. Sin. 2012;33:1069–1079. doi: 10.1038/aps.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]