Fig. 7.
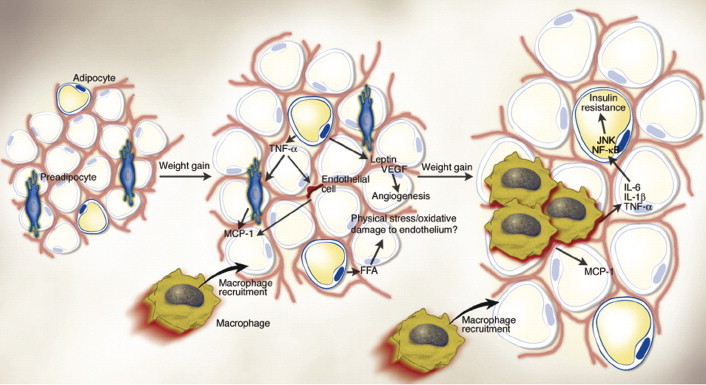
Obese adipose tissue is characterized by inflammation and progressive infiltration by macrophages as obesity develops. Changes in adipocyte and fat pad size led to physical changes in the surrounding area and modifications of the paracrine function of the adipocyte. For example, in obesity, adipocytes begin to secrete low levels of TNF-α, which can stimulate preadipocytes to produce monocyte chemoattractant protein-1 (MCP-1). Similarly, endothelial cells also secrete MCP-1 in response to cytokines. Thus, either preadipocytes or endothelial cells could be responsible for attracting macrophages to adipose tissue. The early timing of MCP-1 expression prior to that of other macrophage markers during the development of obesity also supports the idea that it is produced initially by cells other than macrophages. Increased secretion of leptin (and/or decreased production of adiponectin) by adipocytes may also contribute to macrophage accumulation by stimulating transport of macrophages to adipose tissue and promoting adhesion of macrophages to endothelial cells, respectively. It is conceivable, also, that physical damage to the endothelium, caused either by sheer size changes and crowding or oxidative damage resulting from an increasingly lipolytic environment, could also play a role in macrophage recruitment, similar to that seen in atherosclerosis. Whatever the initial stimulus to recruit macrophages into adipose tissue is, once these cells are present and active, they, along with adipocytes and other cell types, could perpetuate a vicious cycle of macrophage recruitment, production of inflammatory cytokines, and impairment of adipocyte function. (From Wellen et al., 2003).
