Fig. 1.
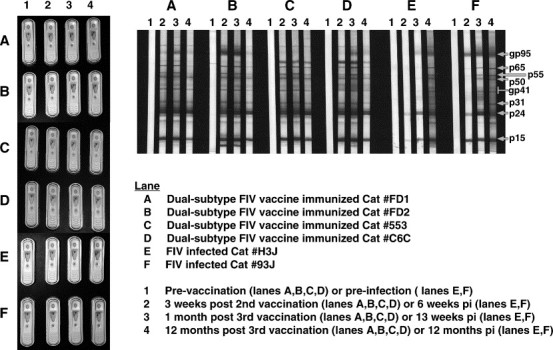
FIV immunoblot and commercial FIV test results of cats immunized with the dual-subtype FIV vaccine or experimentally infected with FIVPET (20 CID50). Sera tested for FIV antibodies were from cats (two each) experimentally immunized with two different sources of dual-subtype FIV vaccine (immunoblot/Snap Combo lanes A, B, C and D) or experimentally infected with FIV (immunoblot/Snap Combo lanes E and F). These sera were tested for the presence of FIV antibodies using immunoblot analysis and commercial Idexx SnapTM Combo (FeLV and FIV) Test Kit. The immunoblot analysis was performed at serum dilution of 1:100 using a published method (Pu et al., 2001) and the Snap test was performed as recommended by the Idexx Laboratories, Inc. Dual-subtype FIV vaccine produced by Fort Dodge Animal Health commercial company (USDA approved product) (lanes A and B) and those produced by our laboratory (lanes C and D) are shown for comparison. Immunogenicity and efficacy of our dual-subtype FIV vaccine has been previously reported (Pu et al., 2001). Both Snap test and immunoblot results demonstrate that vaccinated cats will develop antibodies reactive to current FIV diagnostics. Both vaccinations induced antibodies to the full spectrum of FIV antigens, including antibodies to the envelope (gp95). Interestingly, the cats immunized with the USDA approved dual-subtype FIV vaccine had more consistent and long-lasting antibodies to the envelope compared to cats immunized with our experimental dual-subtype FIV vaccine (2 of 2 cats vs. 1 of 2 cats positive after 1 year post-vaccination).
