Abstract
CDKL5 deficiency disorder (CDD) is a developmental encephalopathy caused by pathogenic variants in the gene cyclin-dependent kinase-like 5 (CDKL5). This unique disorder includes early infantile onset refractory epilepsy, hypotonia, developmental intellectual and motor disabilities, and cortical visual impairment. We review the clinical presentations and genetic variations in CDD based on a systematic literature review and experience in the CDKL5 Centers of Excellence (COEs). We propose minimum diagnostic criteria. Pathogenic variants include deletions, truncations, splice variants, and missense variants. Pathogenic missense variants occur exclusively within the kinase domain or affect splice sites. The CDKL5 protein is widely expressed in the brain, predominantly in neurons, with roles in cell proliferation, neuronal migration, axonal outgrowth, dendritic morphogenesis and synapse development. The molecular biology of CDD is revealing opportunities in precision therapy, with phase 2 and 3 clinical trials underway or planned to assess disease specific and disease modifying treatments.
Keywords: CDKL5 deficiency disorder, developmental encephalopathy, epileptic encephalopathy, epilepsy genetics, clinical trials
Introduction
Pathological variants in cyclin-dependent kinase-like 5 (CDKL5)1–5 cause CDKL5 deficiency disorder (CDD, OMIM 300203, 300672), a developmental encephalopathy (DE).6 DEs share common constellations of features that extend beyond traditional criteria of autism spectrum disorder or intellectual disability such as treatment resistant epilepsy, movement disorders and autonomic dysfunction. Pathological variants in CDKL5 cause early-life epilepsy in 1 in 40,000 – 60,000 live births,7–9 half to a third as prevalent as Dravet (1:20,000–50,000) 10,11 or Rett (1:10,000 females)12 syndromes. Common features include infantile-onset refractory epilepsy, hypotonia, developmental delay, intellectual disability and visual impairment.13–15 CDD is an X-linked disorder that affects females more than males (~4:1)16 as males with germline variants have no normal CDKL5 gene and may not survive fetal life. CDD was initially identified as the early seizure variant of Rett syndrome, but only 23.7% of females and no males with CDD met criteria for typical or atypical Rett syndrome and diagnosis of atypical Rett syndrome is even rarer in recent clinical experience.13,16,17
The literature on CDD includes case series and data from the International CDKL5 Disorder Database, based on caregiver questionnaires.13,18–22 Prospective data collection is occurring through the Natural History Study for Rett and Rett-related disorders (U54 HD061222; ClinicalTrials.gov: NCT00299312/NCT02738281) and through a clinic based study by the International Foundation for CDKL5 Research (IFCR) Centers of Excellence (COEs). Initial sites were Boston Children’s Hospital, Children’s Hospital Colorado and Cleveland Clinic. The COEs provide comprehensive care and collaborate on research for CDD. The COEs have collected data on > 93 individuals with CDD between 0 to 34 years to inform the typical features and spectrum of CDD.16
CDKL5 protein and molecular biology
CDKL5 is a serine/threonine kinase. The N-terminal catalytic domain starts in exon 2 and the long C-terminus may have a regulatory role.23 CDKL5 is highly expressed in the brain, predominantly in neuronal nuclei and dendrites, with peak expression in early postnatal life, when symptoms typically begin.24–27 The CDKL5 protein has roles in cell proliferation, neuronal migration, axonal outgrowth, dendritic morphogenesis and synapse development and function in the adult brain.28
CDKL5 has multiple transcripts due to alternative splicing in mice and humans. The primary brain isoform is hCDKL5_1.23 Pathogenic missense variants occur exclusively within the catalytic domain except for the recurrent missense variant p.Val718Met which affects splicing.29 A male individual mosaic for this variant followed in our COEs has a “typical” CDD phenotype but has walked independently since the age of 2 years. Somatic mosaicism in probands, perhaps more often in males, and presumed parental mosaicism is described; unaffected parents with a full germline CDKL5 variant have not been described.15,20,30–34 Thus, parental testing is critical to assess variants of uncertain significance in CDKL5. There are no biomarkers nor is there a functional assay for variants of uncertain significance; both would be beneficial to the field.
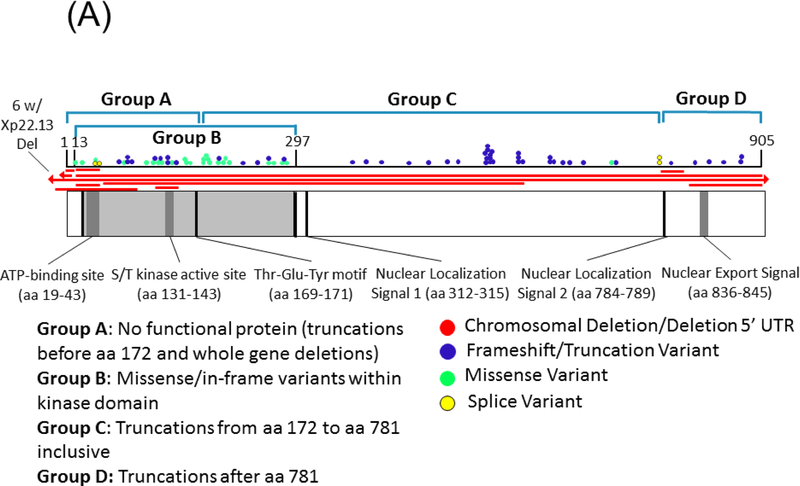
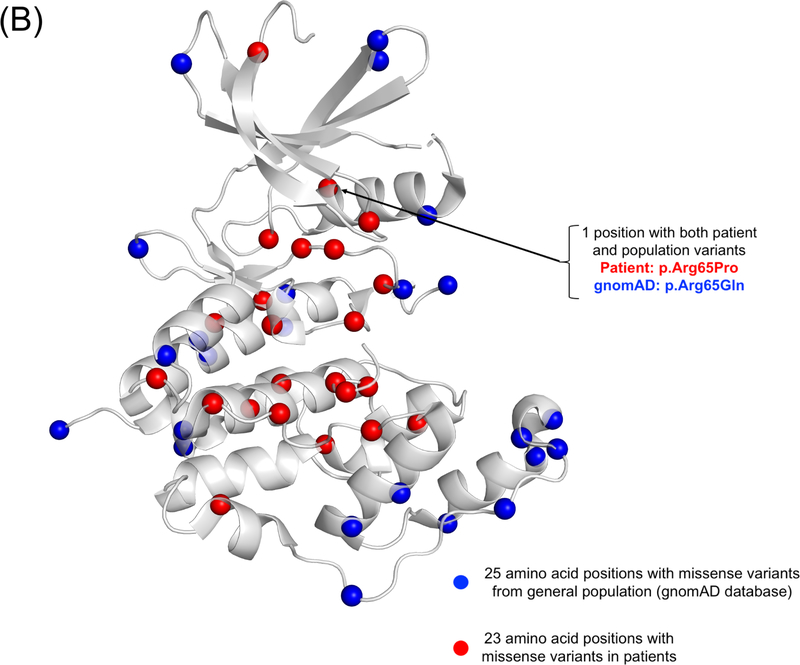
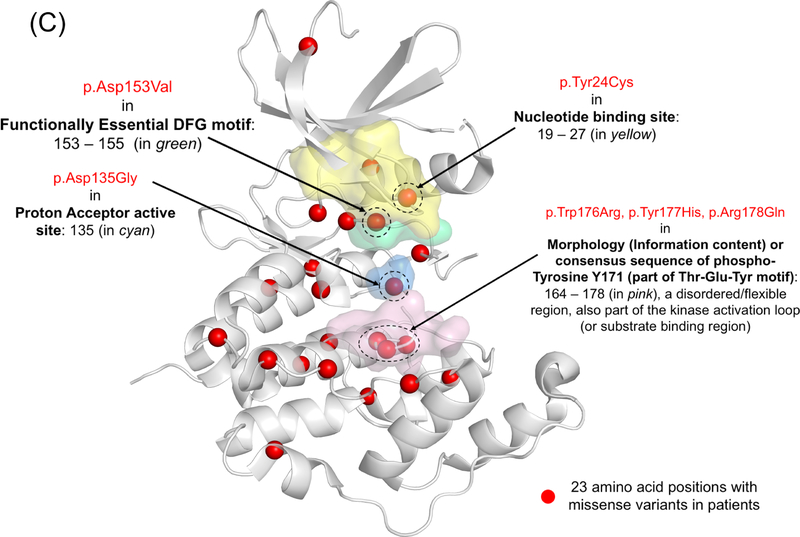
Currently, no evidence supports pathogenic variants in exons 20, 21, and 22 which are part of transcript isoform hCDKL5_5, or within exon 17 which is part of transcript isoform hCDKL5_2.29 The pathogenicity of variants in the 5’ UTR remain uncertain except for deletions extending to include exons 1 and 2.29 Deletions and truncating variants appear to nearly universally cause CDD.29 CDKL5 variants from individuals in the COEs are shown in Figure 1, on a schematic of the protein and on a 3D model along with population variation.
Figure 1.
A) A schematic of the CDKL5 protein with variants from individuals with CDD evaluated in the CDKL5 Centers of Excellence. CDKL5 gene image adapted from prior publication.47 B) 3D protein structure of the CDKL5 gene (Protein Data Bank ID: 4bgq) along with position of population variation (blue spheres) from gnomAD database and variants from the COEs (red spheres). C) Highlight of variants in functional domains in the CDKL5 protein. The missense variants in CDKL5 identified in affected individuals are mapped on the 3D protein structure (protein data bank id: 4bgq) as red spheres (total 23 positions). The yellow-colored region is a nucleotide binding region (aa. 19 – 72) and we observed the disease-associated variant p.Tyr24Cys in this region. The cyan-colored site is a proton acceptor active site (aa. 135) and we observed the disease-associated variant p. Asp135Gly in this site. The green-colored region is a functionally essential DFG motif (aa. 153 – 155) and we observed the disease-associated variant p. Asp153Val in this region. The pink-colored region is the morphology (information content) or consensus sequence of phospho-Tyrosine Y171 (part of Thr-Glu-Tyr motif) (aa. 164 – 178) and we observed the disease-associated variants p.Trp176Arg, p.Tyr177His, p.Arg178Gln in this region.
Individuals with CDKL5 duplications show variable penetrance of macrocephaly and learning disability without epilepsy or magnetic resonance imaging (MRI) abnormalities.35 Neighboring genes are rarely affected in these duplications. This contrasts with other genetic developmental encephalopathies for which duplications cause a different disease than deletions (e.g., MECP2 and FOXG1 disorders).29,36–39 More comprehensive phenome-genome studies of CDKL5 duplication are needed to determine if these duplications are clinically pathogenic.
Molecular studies in rodent models have identified several pathways are altered in CDD, including the AKT/mTOR, AKT/GSK-3b and BDNF-Rac1 signaling pathways and the NGL-1-PSD95 interaction.24,25,27,28,40,41 However, these rodent models demonstrate a behavioral phenotype but lack spontaneous seizure activity.41–43 Dendritic outgrowth and spine development are inconsistently altered in cellular CDD models.41 Mouse model data suggests that CDKL5 expression modulates post-synaptic localization and composition of NMDA receptors.44 CDKL5 influences MeCP2 activity, possibly explaining overlapping features of CDD with Rett syndrome, although the relevance of this in vitro data remains uncertain. Additional CDKL5 substrates include DNMT1, AMPH1, NGL-1, HDAC4, MAP1S, ARHGEF2 and EB2.45,46 A recent review summarized the molecular features of CDD.28
Epilepsy and treatment
Refractory epilepsy severely impacts quality of life and neurodevelopment.14,47 Median age of epilepsy onset is 6 weeks with 90% onset by 3 months.13,14 Eighty percent of children with CDD have daily seizures and 20% have weekly to monthly seizures.48 Less than half (43.6%) of caregivers reported >2 month of sustained seizure freedom.14,47 Among individuals with more than 2 months of seizure freedom (N=71 of 163 families reporting information on seizure freedom), in three quarters of families able to provide additional information this honeymoon period had a median duration of 6 months (range 2.5 months to 6 years) and median onset of 2 years.14 In the COE cohort, 9% of families reported a seizure free period of 1–3 months, 12% 3–6 months, 11% 6–12 months and 13% >12 months. This honeymoon period typically occurs in the first 2 years of life, though some have seizure free periods later in childhood or into their teenage years.16
Three proposed epilepsy stages in CDD include: 1) early onset, at times pharmaco-responsive, 2) epileptic encephalopathy and 3) refractory multifocal and myoclonic epilepsy.49 Infantile spasms are the initial seizure type in 23%, and present at any point in 81% of individuals with CDD.16,50 Evolving epilepsy tends to be generalized or mixed focal and generalized with spasms, tonic, and tonic-clonic seizures most common.16,50 Complex seizure semiology with multiple phases per seizure is common (56%),16,50 including a novel seizure pattern: hypermotor-tonic-spasms sequence.17,51–54 Autonomic changes can be seen intermixed with any of these seizure types, including pupillary dilation, facial flushing, irregular respirations, apneas or hyperventilation.16 While for many individuals refractory epilepsy continues long term, our experience suggests that rare individuals outgrow their epilepsy in childhood and one individual did not have epilepsy onset until 9 years of age (de novo c.1675C>T; p.Arg559Ter).16
Electroencephalograms (EEGs) at onset ranged from hypsarrhythmia to mild abnormalities but more abnormalities in background rhythms and epileptiform activity develop over time.15,18,20,49,53,55,56 Early mild abnormalities that sometimes precede a diffuse encephalopathy included focal delta slowing in the posterior head regions and intermittent generalized slowing.57 Some individuals have hypsarrhythmia and evolution often includes focal or generalized slowing, focal and/or generalized epileptiform activity, and in some cases pseudoperiodic epileptiform discharges.15,18,20,49,53,55,56 Infantile spasms can occur however in the absence of hypsarrhythmia, including with a normal EEG or rare epileptiform activity.16,57 Burst suppression is rare and atypical for neonates with CDD.53
Data on the efficacy of seizure therapies is limited. A review of anti-seizure medication response in 39 individuals with CDD found a responder rate (defined as 50% seizure reduction) to at least one anti-seizure medication of 69% at 3 months, 45% at 6 months and falling to 24% at 12 months.58 Medications with the highest rates of seizure reduction at 3 months included felbamate, vigabatrin, clobazam, valproic acid, steroids, lamotrigine and zonisamide.58 The efficacy of each anti-seizure medication showed large inter-individual variability, with a maximum of 33%, except for felbamate with 3/3 responding at 3 months.58 At 12 months, the responder rate dropped to 0–20% except for 1/3 (33%) still responding to felbamate.58 Exacerbation of seizures occurred with at least one anti-seizure medication in 31% of individuals; most often with carbamazepine (4/15 individuals).58 Our approach in the COEs is to use broad spectrum anti-seizure medications especially when there are generalized seizure types. Overall, 2/39 individuals (5%) became seizure free for >3 years with anti-seizure medication or ketogenic diet.58 The most commonly used anti-seizure medications in CDD were broad spectrum, including clobazam, valproate, topiramate, levetiracetam and vigabatrin and 29.6% of individuals were treated with steroids or ACTH.14 Another study of caregiver perceptions of treatment by survey of 44 individuals with CDD/families reported subjective efficacy (not further defined) in more than 2 individuals to vigabatrin (12/23), clobazam (6/14), sodium valproate (5/27), and levetiracetam (3/27).59 In the Boston Children’s Hospital COE, > 50% reduction in seizures types (excluding epileptic spasms) in more than one individual occurred with the following anti-seizure medications: phenobarbital, clobazam, topiramate, rufinamide and valproic acid.16
Infantile spasms in individuals with CDD are often refractory to first-line therapies. From the parent-entered International CDKL5 Disorder Database, infantile spasms were reported in 33.8% of individuals.14 By contrast, in the COE cohort of 93 individuals with data derived from physicians, spasms occurred in 81% (n=75).50 We hypothesize that the difference in prevalence may result from data collection methods and possible under-diagnosis of infantile spasms if not associated with hypsarrhythmia. Among 18 individuals in the COE cohort with detailed data, median spasm onset was age 4 months (2 weeks to 36 months);60 spasms resolved in only 3/18 individuals (17%) with first line treatments (ACTH or vigabatrin) for epileptic spasms, lower than the ~ 46% response rate at 3 months observed in infantile spasms cohorts.60,61 Since CDD is often diagnosed before spasm onset and other seizure types often occur before spasms, such individuals with CDD are candidates for novel therapies.49,61–65
The ketogenic diet has modest efficacy in treating epilepsy in CDD. The largest cohort reported 104 individuals with CDD treated with median ketogenic diet duration of 17 months and reductions in seizure frequency in 61/104 (58.7%), consistent with data from the Boston Children’s Hospital COE.16,66 Side effects of the ketogenic diet occurred in 31.7% of individuals.66 A smaller cohort of 12 individuals with CDD reported that 2 (17%) had a significant reduction in seizures for >6 months and 1 (8%) for >1 year.58 Behavior improvements were reported including improved alertness in 19/104 (18%) on the ketogenic diet while worsening motor skills and social interactions were reported in 5.8%.66 Ketogenic diet was most often discontinued due to lack of long-term efficacy. These retrospective observational reports did not provide data on diet ratios, ketone levels, efficacy for different seizures types, percent reduction in seizures, or duration of efficacy. Notably, few individuals were treated with ketogenic diet in the first year of life and its efficacy and tolerability in this CDD group remains unknown.
Palliative surgeries for refractory epilepsy include vagus nerve stimulation (VNS) and corpus callosotomy. Among 220 individuals with CDD with parent-entered data, 17% had a VNS implanted and 69% of parents reported reduced seizure frequency.67 These data are consistent with a case report of benefit68 and Boston Children’s Hospital COE reports improvement in 5/6 individuals.16,69 There are no reports of response to corpus callosotomy in the literature and limited experience in the COEs but no response in one individual.16 In the International CDKL5 Disorder Database at least 7/10 individuals had some improvement in seizures following corpus callosotomy of whom two had a longer than 6 month period of seizure freedom (unpublished data).
Development
All individuals with CDD have severe global developmental delays and intellectual disability, though regression is rare except with worsening of seizures or epileptic encephalopathy.13,15,18–20,70–73 Individuals with CDD achieve gross motor milestones at a slowed pace compared to normal. Assessing in girls for whom there are more data, independent walking was attained by 22–23%, raking grasp by 49% by 5 years and pincer grasp by only 13% at any time point.47,71 Using time to event analysis, just under half of individuals could babble by 6 years (43/97 or 44%), and just under a quarter of subjects could speak single words by 7 years of age (17/105 or 16%).47 Spoken language, signs or abstract symbols were produced by 26% of females with CDD (0% of males) and 7.5% of females with CDD spoke in sentences.71 The most common communication modalities were body language, facial expressions, and simple sounds and gestures.71 Use of non-verbal communication devices such as switches and eye gaze technology based communication is often limited by cortical visual impairment, but can be used by some individuals with CDD.16 Autistic features are commonly reported but autism spectrum disorder is infrequently diagnosed due to global developmental impairments.15,18–21,73–76 A diagnosis of autism spectrum disorder has been observed rarely in the COEs.60 Overall, males were reportedly more severely affected than females, though our COE experience does not suggest a striking difference in phenotype.13,16,47 Males can have a milder phenotype.16
Movement disorders
Hand stereotypies were reported in 80% of individuals, and 59% of females and 12.5% of males achieve functional hand use which may be limited by stereotypies.13 The hand stereotypies that we have observed are more consistent with self-stimulatory behavior versus the type of hand stereotypies observed in Rett syndrome.16 Repetitive leg crossing is also commonly observed.16 We lack data on other movement disorders although the COEs have observed episodic or persistent, and occasionally severe, choreoathetosis, akathisia, dystonia, and parkinsonian features.16 Movement disorders may worsen when individuals achieve temporary seizure control.16 At times this may be attributed to polytherapy with anti-seizure medications, improving with reduction in number of anti-seizure medications.16
Physical exam findings
Hypotonia is a nearly universal feature.14,15 Cortical visual impairment is common, occurring in at least 75% of individuals,16 with reports of poor eye contact and lack of visual tracking with an otherwise normal ophthalmologic exam.15,17,18,20,47,72,73 Rotatory and horizontal nystagmus, dysconjugate gaze, abnormal fixation, and reduced or absent optokinetic nystagmus (OKN) response are features observed in individuals with visual impairment. Microcephaly and deceleration of head growth occurs in less than 10% of individuals.13,15,18–21,72,73 Subtle dysmorphic features include deep set eyes, broad or high forehead, prominent lips, deep philtrum, puffy phalanges and tapered fingers.13,15,20,70,73,74 Movement disorders have also been observed as above.
Neuroimaging
Neuroimaging has not yet been systematically reported in individuals with CDD, although case reports document normal brain anatomy or less often, show cortical atrophy or T2/fluid attenuated inversion recovery hyperintensities in the white matter. 15,18–20,49,53,55,56,70,72,73,75,77
Neuropatholgy findings
There is very little literature describing the neuropathological findings in individuals with CDD. One case report described the brain as the sole organ with abnormalities in a post mortem examination.78 In addition to brain and cerebellar atrophy and ventricular enlargement, microscopic examination of the brain revealed gliosis in the cerebral cortex with preservation of the hexalaminar layers, neuronal heterotopias in the white matter of the cerebellar vermis and gliosis of the cerebellar cortex with loss of Purkinje cells and axonal torpedoes.78 Perivascular lymphocytes and axonal swelling in the anterior horn were the main findings in the spinal cord.78 This child had a pathogenic splice variant c.2277–2A>G, predicted to destroy the splice acceptor site of exon 16.78
Other comorbidities
Gastrointestinal symptoms were reported by parents in up to 86.5% in the International CDKL5 Disorder Database (122/141), most often constipation (70.9%), reflux (64.1%), or air swallowing (27.1%).13,48 Orthopedic complications of hypotonia include scoliosis (68.5% by 10 years).13,48 Dysphagia is common and may require gastrostomy. While 79.3% of individuals with CDD in the International CDKL5 Disorder Database fed orally and 20.7% were exclusively fed by gastrostomy or nasogastric tube, some required supplemental tube feedings and only 5.3% were able to eat and drink independently. Notably, ~33% of individuals treated with the ketogenic diet had a gastrostomy; a similar percentage, 11/36 (31%), had gastrostomy in a caregiver survey of individuals with CDD.66 Sleep difficulties are very common, reported by parents in over 85% of individuals, sometimes dubbed “all night parties.” 13,48 Night waking was reported in 72/123 individuals (58.5%).48The odds of sleep problems were highest in the 5–10 year age group compared to those aged less than 5 years.48 Using the Child Health Sleep Questionnaire (CSHQ), the team at Children’s Hospital Colorado found significantly abnormal sleep maintenance and duration.79 Abnormal sleep duration was reported in 63% of individuals with CDD compared to age based norms, and the mean score for waking once per night and more than once per night were elevated (2.45 and 2.25 respectively, p<0.001 for both).79 Breathing abnormalities include hyperventilation reported in 13.6% of individuals, breath holding in 26.4%, and aspiration in 22.6%.48 Parents have expressed concerns about cardiac arrhythmias, and one study by caregiver survey reported arrhythmia in 1½9 individuals with CDD who underwent ECG.59 Arrhythmias have not however been confirmed in the COEs, and this is an area of current investigation.16 Sudden unexpected death in epilepsy (SUDEP) may occur but in large cohorts the frequency of CDD is much lower than Dravet syndrome or SCN8A- related epilepsy given the frequencies of these disorders.80–82 However, the high seizure frequency and severity suggests that individuals with CDD are at high risk of SUDEP, with daily and often nocturnal tonic or tonic-clonic seizures.83 Metabolic abnormalities are rare; a boy with CDD had transient methylmalonic acidemia but the concurrence may be coincidental.84
Clinical criteria
We propose minimum CDD diagnostic criteria to include a pathogenic or likely pathogenic variant in the CDKL5 gene along with motor and cognitive developmental delays and epilepsy with onset in the first year of life. We recognize that some patients with CDKL5 deficiency may be atypical and not meet these formal criteria. Table 1 includes a list of common clinical features and what we determine to be the minimum diagnostic criteria.
Table 1.
Common clinical characteristics and proposed minimal diagnostic criteria.
| Common clinical characteristics | Proposed minimal diagnostic criteria |
|---|---|
| • Epilepsy, early onset and refractory • Severe global developmental delay • Intellectual disability • Hypotonia • Cortical visual impairment • Sleep disturbance • Dyskinetic movements, • Autonomic and breathing disturbances • GI disturbances (reflux, constipation) • Dysphagia |
• A pathogenic or likely pathogenic variant in the CDKL5 gene • Motor and cognitive developmental delays • Epilepsy with onset in the first year of life |
Genotype-phenotype correlations
Genotype-phenotype correlations are limited. Compared to individuals with truncating variants, those with pathogenic missense variants in the ATP binding site had a milder disorder, some with ability to walk unaided, better hand use, and less refractory epilepsy.85 One individual in the COE cohort with a missense variant, p.Tyr24Cys, in the ATP binding site has refractory epilepsy but is making more developmental progress than most individuals with CDD and lacks cortical visual impairment. Another study found that females with late truncating variants after amino acid 781 had better gross motor, hand function and communication milestones than earlier truncating variants.47,71 Seizure frequency was lower in individuals with truncating variants between amino acid 172 and 781 compared to those with no functional protein (incidence rate ratio 0.57; 95% confidence interval 0.35 – 0.93).14 The influence of somatic CDKL5 mosaicism on clinical phenotype is unknown.
Clinical trials and treatments suggested from animal studies
The ultimate goal of understanding the genetics and molecular biology of CDD is to establish precision therapies, targeting the underlying biologic pathways, although the complex biology of CDD makes this challenging. This may include small molecules or perhaps genetic/genomic treatment approaches. The hope is that these therapies may be more effective than currently available treatments.
An open label phase 2 clinical trial of cannabidiol in CDD and three other early life genetic epileptic encephalopathies suggested improvement in frequency of motor seizures >3 seconds in duration.86 The CDD group had a median reduction in motor seizures from median 66.4 per 28 days [IQR 25.9 – 212.0] to 35.8 [IQR 8.9 – 141.6] at 12 weeks, with stable frequency at 48 weeks.86
A phase 2 randomized, placebo-controlled crossover study of Ataluren, a medication that targets pathogenic nonsense variants in other genetic diseases, is in process in CDD (NCT02758626)) but results are not yet available. Another phase 2 trial is being initiated for TAK-935, a novel medication that modulates the NMDA receptor system (NCT03694275).
Ganaxolone, a synthetic methyl derivative of allopregnanolone, is a neurosteroid for which there have been previous trials in the epilepsies including for infantile spasms, status epilepticus, and protocadherin 19-related epilepsy. A phase 2 open-label clinical study is completed, and a phase 3 randomized, placebo-controlled study is ongoing in CDD (NCT03572933). CDKL5 regulates the interaction of IQ motif containing GTPase activating protein 1 with microtubule plus end tracking protein cytoplasmic linker protein 170 (CLIP170), disrupting microtubule dynamics in CDD.82 Pregnenolone, another neurosteroid, restores microtubule association of CLIP170 in CDKL5-deficient neurons, rescuing morphologic defects.82
Molecular pathway abnormalities in CDD rodent models suggest additional possible therapies. Dysregulation in the GSK3-beta pathway in Cdkl5 knockout mouse model led to treatment with a GSK3-beta inhibitor, Tideglusib.40 Treatment during the juvenile period improved hippocampal development and hippocampus-dependent behaviors, whereas treatment in adult mice was not beneficial. Reduced expression of the GluA2 subunit of the AMPA-R was identified in CDKL5 knockout mice.88 Treatment of the mice with the antidepressant tianeptine normalized the expression of membrane inserted AMPA-Rs containing GluA2.88 Treatment of rodents with IGF-1, which activates the AKT/mTOR pathway, rescued dendritic spine instability.89
Protein substitution therapy has been evaluated in animal models with promising results, though feasibility and timeframe to bring this approach to human trials is uncertain.90 Novel therapeutic approaches including genome editing, RNA-based therapeutics and gene therapy are being strongly considered.
Conclusions
The “typical” individual with CDD, defined by having a pathogenic gene variant that impairs CDKL5 function, is characterized by onset of treatment-resistant epilepsy and severe cognitive and motor developmental delays. Epilepsy usually begins in the first three months of life and includes tonic seizures, epileptic spasms without hypsarrhythmia, a seizure-free honeymoon period around 1–2 years old that may last up to 12 months, followed by multiple (2+) seizure types including sequences of mixed seizure type; cortical visual impairment associated with rotatory or horizontal nystagmus, dysconjugate gaze and abnormal fixation; global motor delays with hypotonia and severe impairment of hand function. Permanent regressions or progressive deterioration of neurological function is rare. Other commonly associated features of individuals with CDD include dyskinetic movements, sleep disturbances, autonomic and breathing disturbances, and GI disturbances. We propose minimum CDD diagnostic criteria as above recognizing that some individuals with CDKL5 deficiency may be atypical and not meet these formal criteria.
CDD is an epileptic encephalopathy, defined by the International League Against Epilepsy as a disorder in which “the epileptic activity itself may contribute to severe cognitive and behavioral impairments above and beyond what might be expected from the underlying pathology alone and that these may worsen over time”.91 The transient regressions that occur in CDD are consistent with this definition although there is undoubtedly a developmental component as well. Future studies of the natural history of CDD will better define the role of seizures, interictal epileptiform activity, and anti-seizure medications as factors that may adversely affect these children. We hope that increased preclinical studies to define the molecular consequences of impaired CDKL5 and advances in novel, targeted drug development and molecular biology and genetic approaches will radically transform the prognosis for children with CDD.
Acknowledgments
Funding and Acknowledgements: This work was supported by the National Institute of Neurologic Disorders and Stroke (U54 HD061222, 1K23 NS107646–01 and 1K12NS089417– 01), Rocky Mountain Rett Association, International Foundation for CDKL5 Research, the Ponzio Family Chair in Neurology Research and NHMRC Senior Research Fellowship (#1117105). Drs. Walter E. Kaufmann and Sumit Parikh also participated in data collection by the International Foundation for CDKL5 Research Centers of Excellence.
Abbreviations:
- CDKL5
Cyclin dependent kinase-like 5
- CDD
CDKL5 deficiency disorder
- COE
International Foundation for CDKL5 Research Center of Excellence
Footnotes
Declaration of interest:
Heather E. Olson: none
Scott T. Demarest: Consulting for Upsher-Smith and BioMarin. All remuneration has been made to his department.
Elia M. Pestana Knight: None
Lindsay C. Swanson: None
Sumaiya Iqbal: None
Dennis Lal: None
Helen Leonard: None
J. Helen Cross: J Helen Cross has participated as a clinical investigator for Zogenix, GW Pharma, Marinius Pharmaceuticals and Vitaflo. She has been a member of advisory boards and speaker for Eisai, GW Pharma, Nutricia and Zogenix. All remuneration has been made to her department.
Orin Devinsky: Consultancy/advisory: Privateer Holdings/Tilray, Egg Rock/Papa & Barkley, Receptor Life Sciences, Empatica, Tevard, Engage, Rettco, Pairnomix/Q-state, Zogenix and GW Pharmaceuticals.
Tim A. Benke: Consultancy for AveXis, Ovid, Takeda and Marinus. All remuneration has been made to his department.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Wolf P, Haas HL. Effects of diazepines and barbiturates on hippocampal recurrent inhibition. Archives of Pharmacology. 1977;299:211–218. [DOI] [PubMed] [Google Scholar]
- 2.Weaving LS, Christodoulou J, Williamson SL, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am.J Hum. Genet 2004;75(6):1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laroche S. What can the long-term potentiation procedure tell us about the neural mechanisms of learning and memory? In: Will BE, Schmitt P, Dalrymple-Alford JC, eds. Brain plasticity, learning and memory. Advances in behavioral biology, vol. 28 New York: Plenum Press; 1985:139–155. [Google Scholar]
- 4.Bahi-Buisson N, Villeneuve N, Caietta E, et al. Recurrent mutations in the CDKL5 gene: genotype-phenotype relationships. Am.JMed.Genet.A 2012;158A(7):1612–1619. [DOI] [PubMed] [Google Scholar]
- 5.Majewska MD, Bell JA. Ascorbic acid protects neurons from injury induced by glutamate and NMDA. Neuropharmacology and Neurotoxicology. 1991. [DOI] [PubMed] [Google Scholar]
- 6.Paciorkowski AR, Seltzer LE, Neul JL. Developmental Encephalopathies In: Swaiman KF, Ashwal S, Ferriero DM, et al. , eds. Swaiman’s Pediatric Neurology. 6 ed. Philadelphia: Mosby; 2018:242–248. [Google Scholar]
- 7.Lindy AS, Stosser MB, Butler E, et al. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia. 2018;59(5):1062–1071. [DOI] [PubMed] [Google Scholar]
- 8.Kothur K, Holman K, Farnsworth E, et al. Diagnostic yield of targeted massively parallel sequencing in children with epileptic encephalopathy. Seizure. 2018;59:132–140. [DOI] [PubMed] [Google Scholar]
- 9.Symonds JD, Zuberi SM, Vincent A, et al. The Genetic and Autoimmune Childhood Epilepsy (GACE) Study. American Epilpesy Society; 2017, 2017; Washington, D.C. [Google Scholar]
- 10.Rosander C, Hallbook T. Dravet syndrome in Sweden: a population-based study. Dev Med Child Neurol. 2015;57(7):628–633. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Sullivan J, McDaniel SS, et al. Incidence of Dravet Syndrome in a US Population. Pediatrics. 2015;136(5):e1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurvick CL, de Klerk N, Bower C, et al. Rett syndrome in Australia: a review of the epidemiology. J Pediatr. 2006;148(3):347–352. [DOI] [PubMed] [Google Scholar]
- 13.Fehr S, Wilson M, Downs J, et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur J Hum Genet. 2013;21(3):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fehr S, Wong K, Chin R, et al. Seizure variables and their relationship to genotype and functional abilities in the CDKL5 disorder. Neurology. 2016;87(21):2206–2213. [DOI] [PubMed] [Google Scholar]
- 15.Olson HE, Poduri A. CDKL5 mutations in early onset epilepsy: Case report and review of the literature. Journal of Pediatric Epilepsy. 2012(1): 151–159. [Google Scholar]
- 16.Olson HE, Demarest S, Pestana-Knight E, Benke TA. Clinical experience with CDKL5 Deficiency Disorder in the Centers of Excellence. Unpublished data. 2018. [Google Scholar]
- 17.Artuso R, Mencarelli MA, Polli R, et al. Early-onset seizure variant of Rett syndrome: definition of the clinical diagnostic criteria. Brain Dev. 2010;32(1):17–24. [DOI] [PubMed] [Google Scholar]
- 18.Bahi-Buisson N, Nectoux J, Rosas-Vargas H, et al. Key clinical features to identify girls with CDKL5 mutations. Brain. 2008;131(Pt 10):2647–2661. [DOI] [PubMed] [Google Scholar]
- 19.Liang JS, Shimojima K, Takayama R, et al. CDKL5 alterations lead to early epileptic encephalopathy in both genders. Epilepsia. 2011;52(10):1835–1842. [DOI] [PubMed] [Google Scholar]
- 20.Mei D, Marini C, Novara F, et al. Xp22.3 genomic deletions involving the CDKL5 gene in girls with early onset epileptic encephalopathy. Epilepsia. 2010;51(4):647–654. [DOI] [PubMed] [Google Scholar]
- 21.Nemos C, Lambert L, Giuliano F, et al. Mutational spectrum of CDKL5 in early-onset encephalopathies: a study of a large collection of French patients and review of the literature. Clin Genet. 2009;76(4):357–371. [DOI] [PubMed] [Google Scholar]
- 22.Tao J, Van Esch H, Hagedorn-Greiwe M, et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet. 2004;75(6):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hector RD, Dando O, Landsberger N, et al. Characterisation of CDKL5 Transcript Isoforms in Human and Mouse. PLoS One. 2016;11(6):e0157758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Zhu YC, Yu J, et al. CDKL5, a protein associated with rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. JNeurosci. 2010;30(38):12777–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricciardi S, Ungaro F, Hambrock M, et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol. 2012;14(9):911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusconi L, Salvatoni L, Giudici L, et al. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem. 2008;283(44):30101–30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu YC, Li D, Wang L, et al. Palmitoylation-dependent CDKL5-PSD-95 interaction regulates synaptic targeting of CDKL5 and dendritic spine development. Proc Natl Acad Sci US A. 2013;110(22):9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu YC, Xiong ZQ. Molecular and Synaptic Bases of CDKL5 Disorder. Dev Neurobiol. 2018. [DOI] [PubMed] [Google Scholar]
- 29.Hector RD, Kalscheuer VM, Hennig F, et al. CDKL5 variants: Improving our understanding of a rare neurologic disorder. Neurol Genet. 2017;3(6):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato T, Morisada N, Nagase H, et al. Somatic mosaicism of a CDKL5 mutation identified by next-generation sequencing. Brain Dev. 2015;37(9):911–915. [DOI] [PubMed] [Google Scholar]
- 31.Hagebeuk EE, Marcelis CL, Alders M, Kaspers A, de Weerd AW. Two Siblings With a CDKL5 Mutation: Genotype and Phenotype Evaluation. J Child Neurol. 2015;30(11): 1515–1519. [DOI] [PubMed] [Google Scholar]
- 32.Stosser MB, Lindy AS, Butler E, et al. High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genet Med. 2018;20(4):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartnik M, Derwinska K, Gos M, et al. Early-onset seizures due to mosaic exonic deletions of CDKL5 in a male and two females. Genet Med. 2011;13(5):447–452. [DOI] [PubMed] [Google Scholar]
- 34.Masliah-Plachon J, Auvin S, Nectoux J, Fichou Y, Chelly J, Bienvenu T. Somatic mosaicism for a CDKL5 mutation as an epileptic encephalopathy in males. Am J Med Genet A. 2010;152A(8):2110–2111. [DOI] [PubMed] [Google Scholar]
- 35.Szafranski P, Golla S, Jin W, et al. Neurodevelopmental and neurobehavioral characteristics in males and females with CDKL5 duplications. Eur J Hum Genet. 2015;23(7): 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seltzer LE, Ma M, Ahmed S, et al. Epilepsy and outcome in FOXG1-related disorders. Epilepsia. 2014;55(8):1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunetti-Pierri N, Paciorkowski AR, Ciccone R, et al. Duplications of FOXG1 in 14q12 are associated with developmental epilepsy, mental retardation, and severe speech impairment. Eur J Hum Genet. 2011;19(1):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim Z, Downs J, Wong K, Ellaway C, Leonard H. Expanding the clinical picture of the MECP2 Duplication syndrome. Clin Genet. 2017;91(4):557–563. [DOI] [PubMed] [Google Scholar]
- 39.Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet A. 2010;152A(5):1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs C, Rimondini R, Viggiano R, et al. Inhibition of GSK3beta rescues hippocampal development and learning in a mouse model of CDKL5 disorder. Neurobiol Dis. 2015;82:298–310. [DOI] [PubMed] [Google Scholar]
- 41.Zhou A, Han S, Zhou ZJ. Molecular and genetic insights into an infantile epileptic encephalopathy - CDKL5 disorder. Front Biol (Beijing). 2017;12(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuda K, Takao K, Watanabe A, Miyakawa T, Mizuguchi M, Tanaka T. Comprehensive behavioral analysis of the Cdkl5 knockout mice revealed significant enhancement in anxiety- and fear-related behaviors and impairment in both acquisition and long-term retention of spatial reference memory. PLoS One. 2018;13(4):e0196587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang IT, Allen M, Goffin D, et al. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci U S A. 2012;109(52):21516–21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuda K, Kobayashi S, Fukaya M, et al. CDKL5 controls postsynaptic localization of GluN2B-containing NMDA receptors in the hippocampus and regulates seizure susceptibility. Neurobiol Dis. 2017;106:158–170. [DOI] [PubMed] [Google Scholar]
- 45.Baltussen LL, Negraes PD, Silvestre M, et al. Chemical genetic identification of CDKL5 substrates reveals its role in neuronal microtubule dynamics. EMBO J. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trazzi S, Fuchs C, Viggiano R, et al. HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Hum Mol Genet. 2016;25(18):3887–3907. [DOI] [PubMed] [Google Scholar]
- 47.Fehr S, Leonard H, Ho G, et al. There is variability in the attainment of developmental milestones in the CDKL5 disorder. J Neurodev Disord. 2015;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mangatt M, Wong K, Anderson B, et al. Prevalence and onset of comorbidities in the CDKL5 disorder differ from Rett syndrome. Orphanet J Rare Dis. 2016;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahi-Buisson N, Kaminska A, Boddaert N, et al. The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia. 2008;49(6):1027–1037. [DOI] [PubMed] [Google Scholar]
- 50.Demarest S, Olson HE, Parikh S, Pestana-Knight E, Benke TA. Phenotypic Characterization of CDKL5 Deficiency Syndrome. CDKL5 Forum, London, UK. 2018. [Google Scholar]
- 51.Grosso S, Brogna A, Bazzotti S, Renieri A, Morgese G, Balestri P. Seizures and electroencephalographic findings in CDKL5 mutations: case report and review. Brain Dev. 2007;29(4):239–242. [DOI] [PubMed] [Google Scholar]
- 52.Klein KM, Yendle SC, Harvey AS, et al. A distinctive seizure type in patients with CDKL5 mutations: Hypermotor-tonic-spasms sequence. Neurology. 2011;76(16):1436–1438. [DOI] [PubMed] [Google Scholar]
- 53.Melani F, Mei D, Pisano T, et al. CDKL5 gene-related epileptic encephalopathy: electroclinical findings in the first year of life. Dev Med Child Neurol. 2011;53(4):354–360. [DOI] [PubMed] [Google Scholar]
- 54.Sartori S, Di Rosa G, Polli R, et al. A novel CDKL5 mutation in a 47,XXY boy with the early-onset seizure variant of Rett syndrome. Am J Med Genet A. 2009;149A(2):232–236. [DOI] [PubMed] [Google Scholar]
- 55.Buoni S, Zannolli R, Colamaria V, et al. Myoclonic encephalopathy in the CDKL5 gene mutation. Clin Neurophysiol. 2006;117(1):223–227. [DOI] [PubMed] [Google Scholar]
- 56.Pintaudi M, Baglietto MG, Gaggero R, et al. Clinical and electroencephalographic features in patients with CDKL5 mutations: two new Italian cases and review of the literature. Epilepsy Behav. 2008;12(2):326–331. [DOI] [PubMed] [Google Scholar]
- 57.Poonmaksatit S, Zhang X, Pestana-Knight E. Epilepsy and EEG findings in children with CDKL5 Deficiency Disorder under age 1. American Epilepsy Society Annual Meeting 2018, New Orleans, LA 2018;Abstract 1.140. [Google Scholar]
- 58.Muller A, Helbig I, Jansen C, et al. Retrospective evaluation of low long-term efficacy of antiepileptic drugs and ketogenic diet in 39 patients with CDKL5-related epilepsy. Eur J Paediatr Neurol. 2016;20(1):147–151. [DOI] [PubMed] [Google Scholar]
- 59.Amin S, Majumdar A, Mallick AA, et al. Caregiver's perception of epilepsy treatment, quality of life and comorbidities in an international cohort of CDKL5 patients. Hippokratia. 2017;21(3): 130–135. [PMC free article] [PubMed] [Google Scholar]
- 60.Olson HE, Demarest S, Swanson L, et al. Treatment response of epileptic spasms in CDKL5 disorder. Americal Epilepsy Society Annual Meeting 2016, Houston, TX 2016. [Google Scholar]
- 61.Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79(3):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lux A, Edwards S, Hancock E, et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717. [DOI] [PubMed] [Google Scholar]
- 63.O'Callaghan FJ, Edwards SW, Alber FD, et al. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017;16(1):33–42. [DOI] [PubMed] [Google Scholar]
- 64.Olson HE, Demarest S, Swanson L, et al. Treatment response of epileptic spasms in CDKL5 disorder. Americal Epilepsy Society Annual Meeting 2016, Houston, TX 2016. [Google Scholar]
- 65.Olson HE, Poduri A. CDKL5 mutations in early onset epilepsy: Case report and review of the literature. Journal of Pediatric Epilepsy. 2012;1:151–159. [Google Scholar]
- 66.Lim Z, Wong K, Olson HE, Bergin AM, Downs J, Leonard H. Use of the ketogenic diet to manage refractory epilepsy in CDKL5 disorder: Experience of >100 patients. Epilepsia. 2017;58(8):1415–1422. [DOI] [PubMed] [Google Scholar]
- 67.Lim Z, Wong K, Downs J, Bebbington K, Demarest S, Leonard H. Vagus nerve stimulation for the treatment of refractory epilepsy in the CDKL5 Deficiency Disorder. Epilepsy Res. 2018;146:36–40. [DOI] [PubMed] [Google Scholar]
- 68.Baba S, Sugawara Y, Moriyama K, et al. Amelioration of intractable epilepsy by adjunct vagus nerve stimulation therapy in a girl with a CDKL5 mutation. Brain Dev. 2017;39(4):341–344. [DOI] [PubMed] [Google Scholar]
- 69.Bazin G, Riley K, Swanson L, Bergin AM, Olson HE. Use of Ketogenic diet and vagal nerve stimulators for seizure management in CDKL5 disorder. Translational Neuroscience Center Symposium, Boston Children’s Hospital, Boston, MA 2017. [Google Scholar]
- 70.Archer HL, Evans J, Edwards S, et al. CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J Med Genet. 2006;43(9):729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fehr S, Downs J, Ho G, et al. Functional abilities in children and adults with the CDKL5 disorder. Am J Med Genet A. 2016;170(11):2860–2869. [DOI] [PubMed] [Google Scholar]
- 72.Russo S, Marchi M, Cogliati F, et al. Novel mutations in the CDKL5 gene, predicted effects and associated phenotypes. Neurogenetics. 2009;10(3):241–250. [DOI] [PubMed] [Google Scholar]
- 73.Sartori S, Polli R, Bettella E, et al. Pathogenic role of the X-linked cyclin-dependent kinase-like 5 and aristaless-related homeobox genes in epileptic encephalopathy of unknown etiology with onset in the first year of life. J Child Neurol. 2011;26(6):683–691. [DOI] [PubMed] [Google Scholar]
- 74.Elia M, Falco M, Ferri R, et al. CDKL5 mutations in boys with severe encephalopathy and early-onset intractable epilepsy. Neurology. 2008;71(13):997–999. [DOI] [PubMed] [Google Scholar]
- 75.Fichou Y, Bieth E, Bahi-Buisson N, et al. Re: CDKL5 mutations in boys with severe encephalopathy and early-onset intractable epilepsy. Neurology. 2009;73(1):77–78; author reply 78. [DOI] [PubMed] [Google Scholar]
- 76.Weaving LS, Christodoulou J, Williamson SL, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75(6):1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stalpers XL, Spruijt L, Yntema HG, Verrips A. Clinical phenotype of 5 females with a CDKL5 mutation. J Child Neurol. 2012;27(1):90–93. [DOI] [PubMed] [Google Scholar]
- 78.Paine SM, Munot P, Carmichael J, et al. The neuropathological consequences of CDKL5 mutation. Neuropathol Appl Neurobiol. 2012;38(7):744–747. [DOI] [PubMed] [Google Scholar]
- 79.Friedman S, Moody E, Katz T. Sleep Issues in Patients with CDKL5 Gene Mutation. Pediatric Academic Societies Annual Meeting 2018, Toronto, Canada 2018. [Google Scholar]
- 80.Cooper MS, McIntosh A, Crompton DE, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–47. [DOI] [PubMed] [Google Scholar]
- 81.Johannesen KM, Gardella E, Scheffer I, et al. Early mortality in SCN8A-related epilepsies. Epilepsy Res. 2018;143:79–81. [DOI] [PubMed] [Google Scholar]
- 82.Verducci C, Hussain F, Friedman D, Devinsky O. The North American SUDEP Registry (NASR): Preliminary Descriptive Analysis of SUDEP Cases. American Epilepsy Society Annual Meeting 2018, New Orleans, LA 2018(Abst. 1.425). [Google Scholar]
- 83.Harden C, Tomson T, Gloss D, et al. Practice Guideline Summary: Sudden Unexpected Death in Epilepsy Incidence Rates and Risk Factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017;17(3):180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akamine S, Ishizaki Y, Sakai Y, et al. A male case with CDKL5-associated encephalopathy manifesting transient methylmalonic acidemia. Eur J Med Genet. 2018. [DOI] [PubMed] [Google Scholar]
- 85.Bahi-Buisson N, Villeneuve N, Caietta E, et al. Recurrent mutations in the CDKL5 gene: genotype-phenotype relationships. Am J Med Genet A. 2012;158A(7):1612–1619. [DOI] [PubMed] [Google Scholar]
- 86.Devinsky O, Verducci C, Thiele EA, et al. Open-label use of highly purified CBD (Epidiolex(R)) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–137. [DOI] [PubMed] [Google Scholar]
- 87.Barbiero I, Peroni D, Tramarin M, et al. The neurosteroid pregnenolone reverts microtubule derangement induced by the loss of a functional CDKL5-IQGAP1 complex. Hum Mol Genet. 2017;26(18):3520–3530. [DOI] [PubMed] [Google Scholar]
- 88.Tramarin M, Rusconi L, Pizzamiglio L, et al. The antidepressant tianeptine reverts synaptic AMPA receptor defects caused by deficiency of CDKL5. Hum Mol Genet. 2018;27(12):2052–2063. [DOI] [PubMed] [Google Scholar]
- 89.Della Sala G, Putignano E, Chelini G, et al. Dendritic Spine Instability in a Mouse Model of CDKL5 Disorder Is Rescued by Insulin-like Growth Factor 1. Biol Psychiatry. 2016; 80(4): 302–311. [DOI] [PubMed] [Google Scholar]
- 90.Trazzi S, De Franceschi M, Fuchs C, et al. CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder. Hum Mol Genet. 2018;27(9): 1572–1592. [DOI] [PubMed] [Google Scholar]
- 91.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. [DOI] [PubMed] [Google Scholar]