Fig. 3.
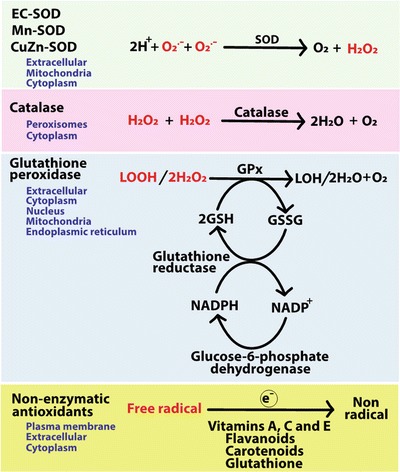
The antioxidant system in cells. Enzymatic and nonenzymatic antioxidants catalyze reactions to neutralize free radicals by donating electrons. Enzymatic antioxidants catalyze reactions to neutralize specific free radicals such that superoxide dismutase (SOD) dismutates superoxide to hydrogen peroxide (H2O2), and catalase and glutathione peroxidase (GPx) convert hydrogen peroxide to water. GPx also converts lipid hyroperoxides (LOOH) to lipid alcohols or aldehydes (LOH). Glutathione reductase replenishes reduced glutathione (GSH) pools from oxidized glutathione (GSSG) using NADPH as reducing equivalents. Nonenzymatic antioxidants such as vitamins, flavonoids and glutathione can also reduce free radicals by donating electrons
