Fig. 5.
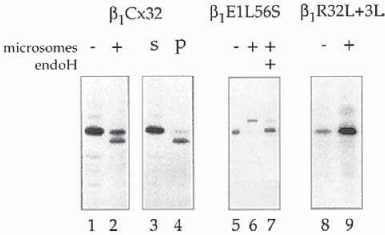
Synthesis of cleaved and full-length membrane-integrated connexins. Translation of connexins in cell-free translation systems supplemented with pancreatic microsomes results in a complete, aberrant processing by the ER resident protease signal peptidase that removes an N-terminal portion including the N-terminal domain, and the first transmembrane spanning domain of connexins (lane 2). In the absence of microsomes no cleavage occurs (lane 1). The cleaved connexins pellet (p) together with the microsomes (lane 4), while the full-length connexins stay in the soluble lysate fraction (s) (lane 3). This result indicates that all membrane-integrated connexin polypeptides are cleaved; and that the full-length connexins also synthesized in the presence of microsomes (lane 2) are synthesized on nonmembrane bound ribosomes, and have failed to insert cotranslationally into the microsomal membranes. Full-length, glycosylated, and membrane integrated connexins were synthesized when an N-glycosylation site was introduced into the first extracellular loop (L56S amino acid exchange) (lane 6). Endoglycosidase H (endoH) removes the carbohydrate sidechain. The electrophoretic mobility of the deglycosylated polypeptides corresponds to the mobility of full-length connexins (lane 7). Full-length, membrane-integrated connexins are also synthesized when the length and hydrophobicity of the first transmembrane spanning domain is increased (R32L + 3L amino acid exchange) (lane 9).
