Table 3.
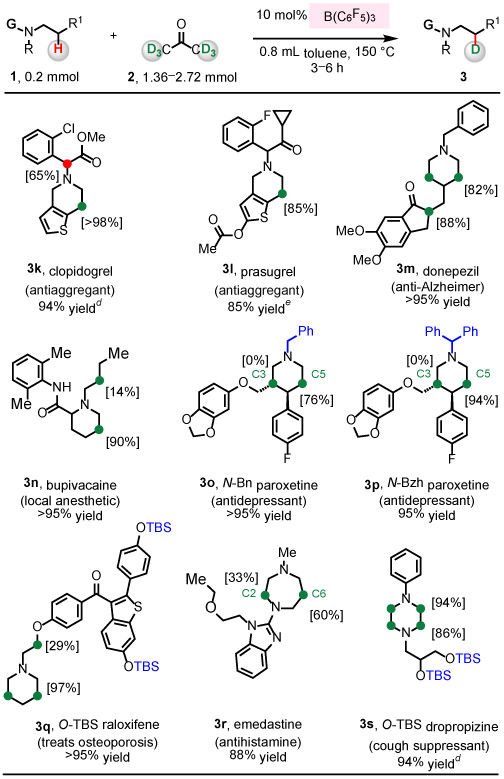 |
Conditions: N-alkylamine (1, 0.2 mmol), acetone-d6 (2, 1.36 mmol), B(C6F5)3 (10 mol%), toluene (0.8 mL), under N2, 150 °C, 3 h.
Yield of isolated and purified product. Deuterium incorporation level was determined by 1H NMR analysis of the isolated and purified product.
Green label indicates sites that are beta to N. Red label is used for any other sites that undergo deuteration.
Conditions: N-alkylamine (1, 0.2 mmol), acetone-d6 (2, 1.4 mmol), B(C6F5)3 (5.0 mol%), toluene (0.8 mL), under N2, 150 °C, 3 h. After the filtration of the crude reaction mixture through a pad of silica gel and removal of volatiles, acetone-d6 (2, 1.4 mmol), B(C6F5)3 (5.0 mol%), and toluene (1.0 mL) were added under N2, and then heated at 150 °C, 3 h.
The reaction was carried out in two batches, using 10 mol% of B(C6F5)3 in the first batch, and 5.0 mol% in the second. For details, see the SI.
