Abstract
The obligate intracellular bacterium Orientia tsutsugamushi is responsible for more than one million cases of scrub typhus annually throughout the Asia-Pacific region. Human infection occurs via the bite of the larval form (chigger) of several species of trombiculid mites. While in some patients the result of infection is a mild, febrile illness, others experience severe complications, which may even be fatal. This review discusses the genome and biology of the causative agent, the changing epidemiology of scrub typhus, the challenges of its diagnosis, and current treatment recommendations.
Keywords: Orientia, Scrub typhus, Trombiculid mite, 56-kDa antigen, Serotypes, Intracellular, Diagnosis, Eschar, Epidemiology, Treatment
Background
During World War II, Allied and Japanese Forces serving in the Asia-Pacific region were afflicted by outbreaks of a serious, acute, febrile disease. More than 16,000 cases were observed among Allied troops and 20,000 cases were recorded in members of the Japanese forces (Bavaro et al. 2005; Philip 1948). Mortality rates were variable, ranging from only 0.6 % in a large outbreak in the Schouten Islands to 35 % in a smaller outbreak in Papua New Guinea (Philip 1948). In some areas, these disease outbreaks were as disabling to a regiment as the combat itself and placed a huge burden on medical facilities (Philip 1948). For example, following a 4-day jungle exercise in Sri Lanka, 756 British soldiers were hospitalized with fever on their return to India (Philip 1948). The disease became known as scrub typhus by the Allied troops, after recognition that infection seemed to be associated with exposure to the secondary vegetation termed “scrub” (Traub and Wisseman 1974).
The disease experienced by the armed forces had been recognized as early as the third century A.D. in China (Fan et al. 1987), but it was first documented in the medical literature as tsutsugamushi disease in 1879 (Nagayo et al. 1917). The name was derived from the Japanese “tsutsuga” meaning illness and “mushi” meaning insect, in reference to the source of infection, the trombiculid mite. The tsutsugamushi disease of Japan and scrub typhus were confirmed to be same disease through studies in laboratory animals (Philip 1948). It has been known by many other descriptive and colloquial names, such as tropical typhus, mite bite fever, Japanese river fever, and Kedani fever (Hayashi 1920; Corbett 1943), but scrub typhus is the name now in common use.
World War II accelerated research into scrub typhus, necessitated by the need to understand and prevent the disease, which was feared by the troops (Philip 1948). This research dwindled after the war, following the discovery that chloramphenicol was an effective treatment (Smadel et al. 1948). However, scrub typhus remains a serious health threat in the Asia-Pacific region. Based on its geographic distribution, an estimated one billion people are at risk of contracting the disease and more than one million cases occur each year (Watt and Parola 2003). Over 10 years ago the World Health Organization identified scrub typhus as “probably one of the most under-diagnosed and under-reported febrile illnesses requiring hospitalization in the region,” and this statement is still valid today (World Health Organization Department of Communicable Disease Surveillance and Response 1999; Paris et al. 2013). The discovery of antibiotic resistant strains, the reemergence of the disease in areas where it had been absent for years and its emergence in new areas, both in the endemic region and beyond, demonstrates that research into scrub typhus is still important. This review summarizes current knowledge.
The Causative Agent of Scrub Typhus
Classification
The causative agent of scrub typhus is , an alpha proteobacterium belonging to the order and the family Rickettsiaceae. When it was first isolated in 1930, it was placed in the genus Rickettsia and named (Nagayo et al. 1930), with a name change to shortly afterward (Ogata 1931). However, it was recognized early on that scrub typhus differed from other rickettsial diseases, in terms of the arthropod vector responsible for infection and clinical presentation (Ashburn and Craig 1908). Later research demonstrated several phenotypic differences between the scrub typhus agent and other species of Rickettsia (Table 16.1).
Table 16.1.
Phenotypic differences between the scrub typhus agent, Orientia ( ) and Rickettsia species
| Feature |
Orientia (Rickettsia) tsutsugamushi
Scrub typhus agent |
Rickettsia species | Reference | |
|---|---|---|---|---|
| Size | Width | 0.5–0.8 μm | 0.2–0.6 μm | Tamura et al. (1991) |
| Length | 1.2–3.0 μm | 0.5–2.5 μm | ||
| Cell wall structure |
Thin inner leaflet Thick outer leaflet |
Thick inner leaflet Thin outer leaflet |
Silverman and Wisseman (1978) | |
| Peptidoglycan and lipopolysaccharide | Absent | Present | Amano et al. (1987) | |
| Capsule layer | Absent | Present | Tamura et al. (1991) | |
| Protein composition (Major protein sizes in kDa) | 110, 80, 70, 60, 54–56, 46–47, 42, 35, 28 and 25 | 155, 120, 49, 32, 27.5, 17.5 and 16.5 | Tamura et al. (1991) | |
| In vitro susceptibility to antibiotics | Tetracyclines | Sensitive | Sensitive | Miyamura et al. (1989) |
| Benzylpenicillin (1 mg/mL) | Resistant | Sensitive | ||
| Ofloxacin (quinolone; 1 μg/mL) | Resistant | Sensitive | ||
Sequencing of the 16S rRNA gene showed that while strains of R. tsutsugamushi shared more than 98.5 % sequence similarity, the level of similarity with other rickettsial species was only 90.2–90.6 % (Ohashi et al. 1995). This genotypic difference, along with the phenotypic differences, provided sufficient evidence for the scrub typhus agent to be reclassified in 1995 into its own genus, Orientia (Tamura et al. 1995). A second member of the Orientia genus has been proposed, although it is not yet officially recognized. The organism was isolated from an Australian patient who became infected while in Dubai in the United Arab Emirates. Sequencing of the 16S rRNA, 56- kDa and 47-kDa genes demonstrated significant genetic diversity (1.5 %, 17.7 %, and 46.9 %, respectively) from strains of O. . Thus, a new species name, O. chuto was proposed, with “chuto” derived from the Japanese for “Middle East” (Izzard et al. 2010).
Genomics
The first full O. genome to be sequenced was the Boryong strain, isolated from a Korean scrub typhus patient (Cho et al. 2007). Shortly after, the genome of the Ikeda strain from a Japanese patient was fully determined (Nakayama et al. 2008) and data from a further eight Whole Genome Shotgun (WGS) projects are now available (Benson et al. 2009). The median genome size of the sequenced genomes is 2.00334 Mb as a single circular chromosome, making the O. tsutsugamushi genome the largest of any member of the order Rickettsiales, with the largest number of protein coding genes (Cho et al. 2007; Nakayama et al. 2008). Although its genome is larger than those of Rickettsia spp., O. has undergone a greater degree of reductive evolution associated with adaptation to an obligate intracellular lifestyle (Andersson and Kurland 1998). It lacks some genes involved in nucleotide metabolism, DNA recombination, DNA repair, and fatty acid biosynthesis, making O. tsutsugamushi more dependent on the functions of its host cell than Rickettsia species in which these genes are conserved (Nakayama et al. 2008).
A unique feature of the O. genome is the high proportion of repetitive sequences present. Repeat sequences (>200 bp) make up around 40 % of the genome, which is 200-fold greater than the density of repeats observed in the R. prowazekii genome and around tenfold greater than in the genomes of most other members of the order Rickettsiales. In fact, the repeat sequence density in the O. tsutsugamushi genome is more than double that of most other bacterial genomes (Cho et al. 2007; Darby et al. 2007; Nakayama et al. 2008, 2010).
The high repeat density is due to extensive amplification of mobile genetic elements that can be classified into three main types: (1) integrative and conjugative elements termed O. amplified genetic elements (OtAGEs) , that spread by conjugative transfer between cells and integrate into the genome; (2) transposable elements (TEs); and (3) short repetitive elements (Nakayama et al. 2008). The OtAGEs make up almost 30 % of the genome (Nakayama et al. 2008, 2010) and comprise a cluster of tra genes for conjugative Type IV secretion systems (T4SSs), flanked on one side by an integrase gene and on the other side by putative effector genes with possible roles in signaling and interaction with the host cell (Cho et al. 2007; Nakayama et al. 2008). Conjugation systems, which facilitate the transfer of DNA between cells, are rare in intracellular bacteria. For example, the R. bellii genome contains a single tra gene operon, similar in sequence and gene organization to the OtAGEs (Ogata et al. 2006) and the plasmid of R. felis contains four tra gene fragments (Ogata et al. 2005). In contrast, the genome of the Boryong strain of O. has 359 tra genes and the Ikeda strain contains 185 remnants of OtAGEs (Cho et al. 2007; Nakayama et al. 2008). The mechanism for the amplification of the OtAGEs is not known but it has been hypothesized that the process may have been important for the adaptive evolution of O. tsutsugamushi, by selecting for components involved in the modulation of host cell functions (Cho et al. 2007; Nakayama et al. 2008). Since many of the genes in the OtAGEs have been pseudogenized by insertions or deletions, the genetic elements are no longer transmissible and their current function is unclear (Cho et al. 2007). The TEs comprise five families of insertion sequence elements, four types of miniature inverted-repeat transposable elements, and a Group II intron, none of which has been identified in the genomes of Rickettsia spp. (Nakayama et al. 2008, 2010). They contribute 13–14 % of the genome sequence, although the amount of each type of TE varies between strains, suggesting significant decay and expansion (Nakayama et al. 2010). The huge amplification of repetitive elements has induced extensive shuffling within the O. genome and as such, little colinearity is observed between strains or with other rickettsial genomes (Nakayama et al. 2008, 2010). However, a set of around 500 genes is conserved between O. strains and five species of and thus may represent the core genes of the Rickettsiaceae family (Nakayama et al. 2010). In addition to intragenomic shuffling, a high rate of homologous recombination has been demonstrated to occur among O. tsutsugamushi strains, which also contributes to the diversification of the population (Sonthayanon et al. 2010). There is also evidence of horizontal gene transfer, through which O. has acquired viral and protist genes encoding ankyrin-repeat proteins and a gene from Cyanobacteria encoding a transposase (Georgiades et al. 2011). Overall, the genomic evolution of O. is unique and may be driven by the population bottlenecks created by its unique life cycle (Nakayama et al. 2008).
Strain Variation
From the early days of research into scrub typhus it was recognized that there were considerable differences in virulence between strains of O. , in both humans and laboratory animals (Bengston 1945). Studies using the complement fixation test (Bengston 1945; Shishido 1964), cross-neutralization (Bell et al. 1946; Bennett et al. 1947), and cross-vaccination (Rights and Smadel 1948) demonstrated serological heterogeneity between strains and three serotypes of Karp, Kato, and Gilliam were defined (Shishido 1964). Following the development of further immunological tools such as immunofluorescence assays (Bozeman and Elisberg 1967; Shirai et al. 1979) and monoclonal antibodies (Eisemann and Osterman 1985; Chang et al. 1990), and more diverse geographical studies (Elisberg et al. 1968), additional variants were identified that differed from the three original serotypes. At least nine major antigenic types are now recognized (Fig. 16.1 and Table 16.2), some of which contain subtypes (Ohashi et al. 1996).
Fig. 16.1.

Major antigenic types of O. . Phylogenetic tree of representative strains of O. tsutsugamushi antigenic types based on the nucleotide sequences of the 56-kDa antigen gene. The tree was constructed from a multiple sequence alignment using the neighbor-joining method, using the algorithm implemented by MEGA6 (Tamura et al. 2013). Clusters relating to the major antigenic types are indicated in bold type. Scale represents base substitutions per site
Table 16.2.
Prevalence of major antigenic types of O.
| Serotype | Prevalence | Comment | |
|---|---|---|---|
| Karp | 39.5 % | Found throughout the endemic area. | |
| JG-related |
JG-v JG |
10.3 % 8.1 % |
Referred to as “Gilliam-type in Japan” based on serological reactivity, although sequences are quite divergent from Gilliam. JG-v is a closely related variant. |
| TA763 | 9.6 % | First identified in Thailand but now found in China, Taiwan, India, Australia, and southeast Asia. May not occur in Japan and Korea. | |
| Kato | Kato | 6.3 % | Found throughout the endemic region. |
| Kt-v | 5.2 % | ||
| Saitama | 5.5 % | Similar to Karp-related strains. Found mostly in Japan but also identified in Korea and China. | |
| Kuroki | 4.1 % | Includes the fully sequenced Boryong strain. | |
| Kawasaki | 4.1 % | Found in Japan and China. | |
| Gilliam | 4.1 % | Prototype strain isolated in Myanmar but subsequently identified in isolates from India and China. | |
| Shimokoshi | 1.1 % | Highly divergent based on 56-kDa antigen gene sequence analysis. | |
Prevalence among collection of 271 strains for which the complete or almost complete sequence of the 56-kDa antigen gene was available (Kelly et al. 2009)
The antigenic diversity of O. is due to variation of the 56-kDa outer membrane protein (Eisemann and Osterman 1985; Stover et al. 1990; Tamura et al. 1985), which is considered to be species specific (Kelly et al. 2009). Characterization of the gene encoding the 56-kDa antigen from different strains demonstrated variation in its length, resulting in the protein size ranging from 516 to 541 amino acids (Ohashi et al. 1992; Kelly et al. 2009). Comparison of different strains showed that throughout the protein sequence there are insertions, deletions, or substitutions of one or more amino acids. However, there were four particular regions in which higher rates of change were observed (variable domains (VD) I–IV) (Fig. 16.2), with protein sequence similarity between strains ranging from more than 75 % to less than 50 % (Ohashi et al. 1992). The diversity of the VDs cannot be attributed to point mutations alone and it is likely that recombination has occurred in these regions. This is supported by the fact that some strains seem to be chimeric. For example, in the Kuroki strain, VD I is similar to the Gilliam strain, VD II is Kuroki specific, VD III is similar to the Karp strain, and VD IV is similar to both Gilliam and Karp (Ohashi et al. 1992).
Fig. 16.2.
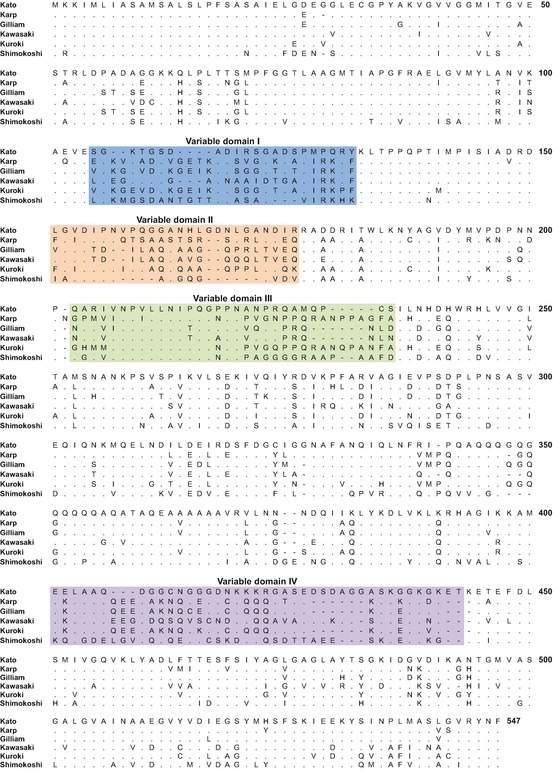
Alignment of 56-kDa antigen sequences. Protein sequences were aligned using the ClustalW algorithm in MEGA version 6 (Tamura et al. 2013). Colored shading indicates the variable domains I–IV
Since gene sequencing has become readily available, determination of the 56-kDa antigen gene sequence has become the major tool for the differentiation and classification of O. strains (Enatsu et al. 1999; Kelly et al. 2009). However, phylogenies based on these sequences are not accurate and different trees are obtained depending on which of the VD sequences is used (Nakayama et al. 2010). More recently, multilocus sequence typing (MLST) schemes based on seven (Sonthayanon et al. 2010; Wongprompitak et al. 2015) or 11 (Nakayama et al. 2010) housekeeping genes have been used for differentiation. A low level of congruence was observed between the MLST and 56-kDa antigen gene sequencing data (Nakayama et al. 2010; Sonthayanon et al. 2010; Wongprompitak et al. 2015), with MLST providing significantly better discrimination between isolates. For example, among a collection of 22 isolates, 15 sequence types were determined using MLST compared with only three antigenic types determined using the single locus method (Sonthayanon et al. 2010). In contrast to the strongly clustered trees created from 56-kDa antigen gene sequences (Fig. 16.1), phylogenies based on MLST data have poor bootstrap support and the limited clustering observed does not correlate with the 56-kDa gene clusters (Wongprompitak et al. 2015). This limited correlation of clusters was also observed when comparing phylogenetic trees created from sequences of the groES and groEL genes with 56-kDa antigen gene phylogenies (Arai et al. 2013). While the 56-kDa antigen gene sequence may be appropriate for investigation of local outbreaks, it is less useful for the phylogenetic analysis of O. , since this antigen gene is under selection pressure from host immune systems (Sonthayanon et al. 2010).
Regardless of which typing method is used, there is little evidence to support the grouping of a specific antigenic or sequence type to a particular geographical location (Enatsu et al. 1999; Kelly et al. 2009; Wongprompitak et al. 2015). However, in some areas, one prevalent antigenic type is observed, which may be linked to the prevalence of a particular mite vector species (Ogawa and Ono 2008). Of the main serotypes, Karp is the most common, accounting for approximately 40 % of all isolates (Kelly et al. 2009). Many of these isolates are described as “Karp related” as they have slight differences in the 56-kDa gene sequence compared to the prototype Karp strain, but the same reading frame is maintained (Kelly et al. 2009). The prevalence of the other serotypes ranges between 1.1 and 10.3 % (Table 16.2). While some cross-reactivity is observed between antibodies raised against one serotype with antigens from another, it is important for diagnostic serological methods to incorporate a mixture of antigens to ensure cases due to an antigenically variant strain of O. are not missed.
Biology of O.
The typical morphological form of an O. tsutsugamushi cell is a short rod or coccobacillus, with a length of 1.2–3.0 μm (Tamura et al. 1991) (Fig. 16.3). It is Gram negative (Tamura et al. 1995), although the Giemsa or Gimenez stain is more useful for observing the organism grown in tissues (Gimenez 1964). Propagation of O. tsutsugamushi must be performed in a biosafety level 3 (BSL3) laboratory (U.S. Department of Health and Human Services 2009) and as it is an obligate intracellular bacterium, its growth in vitro requires a host cell. Successful growth has been achieved in the yolk sac membrane of embryonated chicken eggs (Clancy and Cox 1946) and a variety of cell lines including mouse lymphoblasts (Bozeman et al. 1956), mouse fibroblasts (Urakami et al. 1982), and human endothelial cells (Kim et al. 1999). However, intracellular growth studies of O. tsutsugamushi have been hampered by its slow replication and comparatively low yields in vitro. Studies have shown the bacterial load can be improved by the addition of up to 20 % fetal bovine serum and 0.2–0.8 μg/mL daunorubicin (an inhibitor of host cell replication) to the culture medium, although the cell doubling time is still slow, at around 9 h (Giengkam et al. 2015; Hanson 1987). While cell-free media have been developed for other species that were previously considered to be strictly intracellular, such as and , axenic culture of O. has not yet been possible (Singh et al. 2013).
Fig. 16.3.
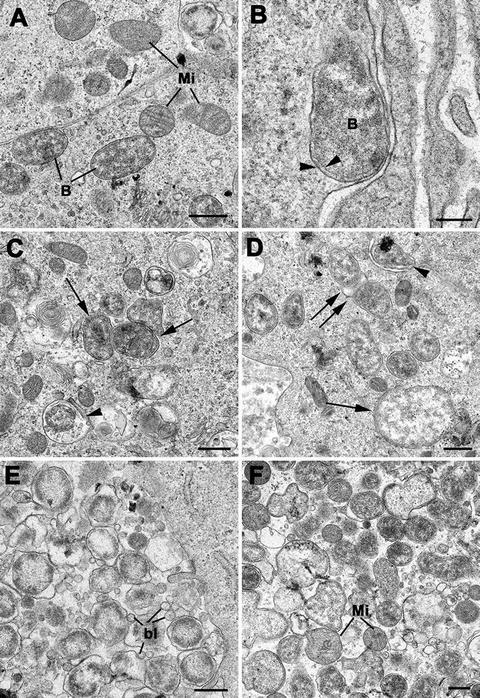
morphology after infection of Vero cells (green monkey kidney epithelial cells, O. tsutsugamushi Thai strain UT76). (Images courtesy of Daniel Paris, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University, Thailand and Centre for Tropical Medicine and Global Health, University of Oxford, UK). (a) Intact cytosolic O. tsutsugamushi (B), in close vicinity to mitochondria (Mi), both with and without cristae. Bar length corresponds to 500 nm. (b) Submembrane location of a single O. tsutsugamushi; the membrane protrusion is suggestive of budding in progress. Distinguishing feature of O. tsutsugamushi—double membrane with double leaflets (arrowheads). Bar length corresponds to 200 nm. (c) Intact O. tsutsugamushi (arrows) and degenerating O. tsutsugamushi in vacuoles (arrowhead). Bar length corresponds to 500 nm. (d) Vero cell with intact and free cytosolic O. tsutsugamushi. In the lower right is a large (approximately 2 mm in diameter) O. tsutsugamushi featuring a double-membrane and diffuse bacterial cytoplasm (arrow). A single O. tsutsugamushi is in the process of budding and potentially invading the adjacent cell (arrowhead). Another O. tsutsugamushi is in the process of mitosis and binary fission (double arrow). Bar length corresponds to 500 nm. (e) Multiple O. tsutsugamushi within a phagosome. There is abundant production of blebs (bl), protrusions and cleaving of the outer membrane of O. tsutsugamushi. Bar length corresponds to 500 nm. (f) O. can appear in various forms and sizes, with condensed or grainy and loose cytoplasm. Note the close vicinity with mitochondria (Mi). Bar length corresponds to 500 nm
The in vivo target cells for human infection by O. tsutsugamushi are initially dendritic cells, monocytes, and macrophages in the skin at the site of the mite bite (Paris et al. 2012). The infection becomes disseminated, initially by migration of monocytes and dendritic cells to regional lymph nodes and via the lymphatic system (Paris et al. 2012), and subsequently via the blood to other organs, where endothelial cells and macrophages are the main bacterial targets (Moron et al. 2001; Keller et al. 2014).
Adherence and uptake of the bacteria by nonphagocytic host cells occurs through the process of endocytosis, which exploits host cell signaling pathways, leading to rearrangement of the host actin cytoskeleton. Several interactions between O. tsutsugamushi ligands and host receptor molecules have been implicated in the adhesion of bacteria to the host. The 56-kDa antigen of O. tsutsugamushi has been shown to bind to syndecan-4, a cell surface heparan sulfate proteoglycan (Kim et al. 2004a), and fibronectin (Cho et al. 2010; Lee et al. 2008). Inhibition of these interactions results in reduced internalization of bacteria by the host (Kim et al. 2004a; Cho et al. 2010; Lee et al. 2008). The bacterial autotransporter protein, ScaC, also binds to fibronectin, although this interaction seems only to enhance bacterial adherence, as it does not result in bacterial invasion (Ha et al. 2011). The formation of the 56-kDa antigen–fibronectin complex leads to engagement of integrin α5β1 molecules at the host cell surface and the subsequent activation of focal adhesion kinase (FAK) and Src tyrosine kinase through tyrosine phosphorylation (Cho et al. 2010). As a result, focal adhesions are formed and focal adhesion signaling adaptor proteins, such as talin and paxillin, are recruited to the site of attachment (Cho et al. 2010). In vitro observations by confocal microscopy have shown that within 10 min of infection, O. tsutsugamushi organisms are surrounded by membrane protrusions from the host cell surface. These are caused by rearrangements of the actin cytoskeleton, induced by the small GTPase, RhoA, which is activated following focal adhesion (Cho et al. 2010).
In addition to activation of the integrin-mediated signaling pathway, O. tsutsugamushi causes a transient activation of the signaling enzyme PLC-γ, which leads to the mobilization of calcium ions (Ca2+) from intracellular calcium stores in the host. As a result, the host cytoplasmic calcium concentration is increased. It is currently unclear what role this plays in O. tsutsugamushi infection, but in vitro, inhibition of PLC-γ reduces bacterial invasion by around 80 %. It is thought that activation of the Ca2+ signaling pathway may occur prior to or in parallel with integrin-mediated signaling and may contribute to the rearrangement of host actin (Ko et al. 2011).
O. tsutsugamushi cells that induce host cytoskeleton rearrangement are internalized by clathrin-dependent endocytosis, similar to several other intracellular pathogens such as spp. and (Chu et al. 2006). Once internalized, bacteria are contained within clathrin-coated vesicles, which move through the endocytic pathway as evidenced by colocalization of O. tsutsugamushi with the early endosome marker EEA1 and late endosome and lysosome marker LAMP2 (Chu et al. 2006). This colocalization is only observed early in the infection process. By 2 h postinfection, the bacteria have escaped from the endocytic compartment and are free in the cytoplasm of the cell (Chu et al. 2006). This process is pH dependent, as demonstrated by studies in which O. tsutsugamushi failed to traffic to late endosomes if the normal acidification of the endosome was prevented (Chu et al. 2006). The mechanism by which the bacteria promote their release from the intracellular compartment is not yet elucidated but it may involve a hemolysin or phospholipase D (Chu et al. 2006; Ge and Rikihisa 2011). The endocytic pathway is not involved during O. tsutsugamushi infection of professional phagocytes such as macrophages (Chu et al. 2006). Instead, the bacteria become internalized within a phagosome, from which some may escape into the host cytoplasm (Rikihisa and Ito 1979).
O. free within the cytoplasm move from the periphery of the cell to the microtubule organizing center in the region around the nucleus, using the microtubule network. Bacterial movement is mediated by the motor protein dynein. This is in contrast to other rickettsiae, which move within the cytoplasm using the host cell actin (Kim et al. 2001). Although intranuclear replication of O. tsutsugamushi has been observed (Urakami et al. 1982; Pongponratn et al. 1998), replication occurs more typically in the perinuclear region of the cytoplasm by binary fission (Urakami et al. 1984) (Fig. 16.3d). As the host cell becomes more heavily infected with O. tsutsugamushi, micro-colonies are observed, which may be biofilm-like structures, aggregated by matrices of the bacterial polysaccharide antigen, NT19 (Lee et al. 2009). In vitro, by 72 h postinfection, O. tsutsugamushi cells are observed throughout the host cell cytoplasm, including at the cell periphery. The bacteria push on the host cell membrane from the inside (Fig. 16.3b, d) and as the infection proceeds, numerous bud-like projections containing bacteria are observed on the host cell surface (Urakami et al. 1984). The bacteria are released from the host cell, either in host membrane coated buds (Ewing et al. 1978) or as free bacteria, following lysis of the host (Rikihisa and Ito 1979). Both forms have been shown to be capable of infecting other new cells, although one form may be more infective than the other for some host cell types. For example, in a study performed in mice, mesothelial cells of the peritoneal cavity were able to phagocytose the host membrane coated vesicles containing O. tsutsugamushi but not uncoated bacteria (Ewing et al. 1978). A similar budding process has been observed in mites infected with O. tsutsugamushi, where bacteria are released from the cells of the mite salivary glands, when triggered by mite feeding (Kadosaka and Kimura 2003).
The interaction of O. tsutsugamushi with its host is a complex process. A study of human monocytes showed that the expression of more than 4500 genes was altered in response to infection by O. tsutsugamushi. The most up-regulated genes (>20 % enrichment) were those involved in the immune response and more than 15 % of these genes were cytokines or chemokines (Tantibhedhyangkul et al. 2011). While a normal host response elicits the production of cytokines, if this production is excessive it can cause a severe systemic disorder in the host (Ge and Rikihisa 2011). The hyperproduction of cytokines and chemokines has been observed in a scrub typhus susceptible mouse model (Yun et al. 2005) and in scrub typhus patients, and is likely linked to some of the systemic symptoms observed (Kramme et al. 2009; Chung et al. 2008). Infection with O. tsutsugamushi can result in the subversion of functions within the host cell, which promotes bacterial survival. For example, the process of autophagy plays an important role in both the innate and adaptive immune response against several intracellular pathogens (Choi et al. 2013). Although O. tsutsugamushi induces autophagy, it actively escapes the autophagosomes, therefore its growth is unaffected by the process (Choi et al. 2013; Ko et al. 2013). Whole genome sequencing of O. tsutsugamushi revealed a large number of genes encoding proteins with eukaryotic ankyrin-repeat (Ank) domains (Cho et al. 2007; Nakayama et al. 2008). These proteins have been found in other intracellular bacteria such as Coxiella burnetii and Anaplasma phagocytophilum, in which they have been implicated in the regulation of host cell processes. Recent studies have shown that the O. tsutsugamushi Ank proteins are substrates for the Type I secretion system (Beyer et al. 2015; Min et al. 2014; Viebrock et al. 2014). Several of these proteins traffic to the endoplasmic reticulum of the host cell, where they may be involved in modulation of the host response to infection (Viebrock et al. 2014) and one (Ank9) interacts with the host cell polyubiquitination machinery, although the host cell protein targets are yet to be determined (Beyer et al. 2015). O. tsutsugamushi may also affect the metabolism of its host. For example, in L929 cells, infection with O. tsutsugamushi resulted in altered lipid metabolism, with the accumulation of organelles capable of storing triglycerides, known as lipid droplets (Ogawa et al. 2014). The role of this alteration has not yet been determined, but lipid droplets are essential for the growth of (Cocchiaro et al. 2008), and may also be involved in the growth or survival of O. (Ogawa et al. 2014).
Disease Transmission
Like other rickettsial diseases, scrub typhus is arthropod borne. Its vector is the larval stage of more than 50 species of trombiculid mites belonging to several genera, but species of the genus are considered to be the primary cause of disease transmission in most countries (Lee et al. 2011). These larvae (Fig. 16.4) are commonly referred to as chiggers.
Fig. 16.4.
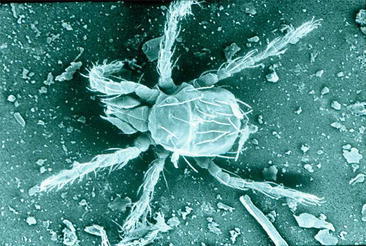
Larval (chigger) stage of the trombiculid mite. Micrograph of an unfed larva of the species . Although not a vector of scrub typhus, this larva is typical of the trombiculid mites. (Image courtesy of Stephen Frances, Vector Surveillance and Control, Australian Army Malaria Institute)
The life cycle of trombiculid mites encompasses four main stages: egg, larva, nymph, and adult (Fig. 16.5). Females lay their eggs loosely in soil over a period of 6–12 weeks, at a rate of one to five eggs per day. After a break of a similar time, a second phase of oviposition takes place. During this whole 3–5 month period, a single female will lay around 400 eggs. The egg stage lasts only 5–7 days, then the shell ruptures, exposing the deutovum (an immature, quiescent form), which develops into the larva within another week. The six-legged larvae that emerge are barely visibly to the naked eye. Within 2 days of emergence, they are ready to feed, with most species attaching to any mammal or bird with which they can make contact. The larvae feed on serum exudate and cellular debris rather than blood, taking 2–12 days to become fully engorged. After detaching from their host, they enter a second quiescent, pupa-like phase (protonymph), and develop into the eight-legged nymph within 7–10 days. The nymphs do not require a host, but instead feed on the eggs of other arthropods and quiescent or deceased soft-bodied insects. Within about 2 weeks, the nymphs enter another quiescent phase (tritonymph) and emerge as adult mites around 2 weeks later. Male adults deposit spermatophores, which are collected by the adult females, and oviposition begins within a fortnight. The life cycle of most mites typically takes 2–3 months, but it may be as long as 8 months in some species. Adult mites live for around 4–6 months, although some have been observed to survive for more than 14 months in the laboratory (Neal and Barnett 1961; Traub and Wisseman 1974; Walker et al. 1975).
Fig. 16.5.

Life cycle of trombiculid mites. The life cycle comprises four main stages (egg, larva, nymph, and adult) with three intermediate, quiescent stages. Humans are an incidental host of the larvae, which more commonly feed on mammals or small birds (Santibáñez et al. 2015)
Since only the larvae bite humans, for the mite to act as a vector for scrub typhus it is essential that the causative bacteria are transmitted trans-stadially to the subsequent nymph and adult stages, then transovarially from the female to the eggs (Burgdorfer and Varma 1967). This results in a new population of infected larvae capable of transmitting disease. The transovarial transmission of O. has been demonstrated and studied in many species of trombiculid mites (Rapmund et al. 1969, 1972; Traub and Wisseman 1974; Roberts and Robinson 1977; Frances et al. 2001; Phasomkusolsil et al. 2009; Shin et al. 2014). This process is very efficient, with transovarial transmission rates (percentage of infected females that transmit the bacteria to their progeny) often as high as 100 %. Filial infection rates (percentage of infected larvae derived from a single infected female) are more variable and seem to vary between parents (Rapmund et al. 1969; Urakami et al. 1994a). In laboratory reared mites, three main patterns of filial infection rates have been observed: (1) all the progeny are infected, (2) all except one or two individuals are infected, and (3) the offspring are mostly uninfected (Rapmund et al. 1969). In some laboratory-reared colonies, filial infection became less efficient over time. Commonly, 100 % of the first generation of larvae from O. infected females was also infected, whereas in later generations, the overall filial infection rate dropped to less than 65 % (Urakami et al. 1994a; Frances et al. 2001; Phasomkusolsil et al. 2009).
In infected mites, O. is distributed throughout the body and found in most tissues, although not all studies have demonstrated its presence in the muscles (Urakami et al. 1994a, b; Wright et al. 1984). Particularly high concentrations of the organism are found in the salivary glands of the larvae and in the salivary glands, excretory bladder, epidermal layer, digestive tissues, and reproductive organs of adults (Wright et al. 1984; Urakami et al. 1994a). Although the bacteria have been observed in the testes of infected adult males, they are not transmitted to the spermatophores (Takahashi et al. 1988; Urakami et al. 1994b), thus only infected females are capable of passing the infection to their offspring. In some species, infection with O. has been shown to affect the development of infected mites. Infected larvae of L. chiangraiensis and L. imphalum were shown to feed and detach from their host quicker than larvae that were not infected. This may be advantageous as the period where the mites are vulnerable to antimite behavior by the host is reduced (Phasomkusolsil et al. 2012). Egg production in several species of mites is higher when the adult females are infected (Roberts et al. 1977; Frances et al. 2001), again conferring an advantage to the mite, by increasing the number of progeny. However, in other species, a reduction in both egg production and hatching is observed in infected mites (Roberts et al. 1977; Phasomkusolsil et al. 2012; Shin et al. 2014), which would be expected to decrease their survival. In some species of mite studied, infection with O. tsutsugamushi alters the sex ratio of the offspring from around 2:1 in favor of females, to exclusively females (Roberts et al. 1977; Shin et al. 2014). This phenomenon has not been observed in uninfected mites.
The primary hosts of the trombiculid mite larvae are wild rodents. Many studies have demonstrated the presence of O. tsutsugamushi in these animals (Jackson et al. 1957; Walker et al. 1973; Glazebrook et al. 1978; Ishikura et al. 1985; Lerdthusnee et al. 2008; Cosson et al. 2015) and the larval mites successfully transmit the bacterium to their rodent hosts (Frances et al. 2001; Lerdthusnee et al. 2002). Although the larval acquisition of O. from an infected animal and subsequent trans-stadial transmission have been demonstrated, the infection is very rarely passed on to the mites’ offspring (Traub et al. 1975; Walker et al. 1975; Takahashi et al. 1994). It is thought that the bacteria may not penetrate the walls of the gut, thus never reaching the correct part of the mite’s body to be transmitted by the transovarial route (Walker et al. 1975; Traub et al. 1975). The reservoir of a disease must be capable of maintaining “a regular or permanent source of infection in nature” (Traub et al. 1975). Since uninfected mites acquiring O. from their hosts are not able to transmit the bacteria to another host, wild rodents are not a reservoir of scrub typhus. Instead, trombiculid mites are considered to be both the disease reservoir and vector. However, rodents are important in the ecology of scrub typhus and it is likely that infected rodents provide the explanation for the occurrence of multiple strains of O. tsutsugamushi within individual larvae (Shirai et al. 1982; Frances et al. 2000).
Epidemiology
Traditionally, the worldwide distribution of scrub typhus has been defined by the ‘ tsutsugamushi triangle.’ This describes the region in which the disease is endemic and encompasses an area of approximately 13 million square kilometers, with the northern most point in Korea and the far east of Russia, reaching to tropical northern Australia in the south and Afghanistan in the west (Paris et al. 2013) (Fig. 16.6). However, in recent years, this concept has been challenged by cases of the disease outside of the endemic region. The aforementioned case of scrub typhus caused by the proposed new species O. chuto was acquired in Dubai in the United Arab Emirates, around 500 km west of the previously recognized area (Izzard et al. 2010) and in 2006, a patient bitten by a leech on a Chilean island developed a scrub typhus-like illness, with an Orientia-like agent confirmed by DNA sequencing (Balcells et al. 2011). If the leech is shown to be an alternative vector for scrub typhus, the distribution of the disease may be increased. A further 3 confirmed cases of scrub typhus have more recently been reported from the same island, in patients that had never travelled outside of Chile, therefore the infection may now be endemic in this region (Weitzel et al. 2016). There have also been reports of scrub typhus cases and seroprevalence studies in which infection with O. was believed to have occurred in Africa (Osuga et al. 1991; Ghorbani et al. 1997; Thiga et al. 2015; Luce-Fedrow et al. 2015). It is important for clinicians in nonendemic countries to consider the disease in travelers with compatible symptoms returning from the endemic region, and in light of these recent cases to be aware that scrub typhus may also be acquired outside of the ‘ tsutsugamushi triangle.’
Fig. 16.6.

Worldwide distribution of scrub typhus. Map indicates the regions in which scrub typhus is known to be endemic (red) and the locations of scrub typhus cases caused by O. chuto (green dot) and an Orientia-like species (yellow dot). Possible locations of scrub typhus infection in Africa are also indicated (blue dots)
Humans become infected with O. via the bite of an infected chigger, when they encroach on the habitats in which the mites are found. Therefore, the epidemiology of scrub typhus is closely linked to the distribution and behavior of the trombiculid mite species that are vectors of the disease. Unsurprisingly, positive correlations have been demonstrated between the number of human scrub typhus cases in an area with both the coincidence of mite vector species (Ishikura et al. 1985; Roh et al. 2014) and the prevalence of O. tsutsugamushi infection in wild rodents (Ishikura et al. 1985; Lin et al. 2014). The description of the disease as scrub typhus is a misnomer as trombiculid mites are not restricted to the scrub vegetation of subtropical regions but are found in a diverse range of climates and habitats. These include subarctic regions, semidesert (Traub and Wisseman 1968), woody vegetation (Traub and Wisseman 1974), deep jungle (Cadigan et al. 1972), and rice paddies (Tanskul et al. 1998). Some of the scrub typhus vector mite species have a very restricted distribution. For example, L. arenicola is only found in Malaysia (Upham et al. 1971). Other species, such as L. deliense, appear to be more adaptable and their distribution is more widespread (Traub and Wisseman 1974). Within the areas in which the mites are found, their distribution can be highly focal. Localized units of mites are termed “ mite islands” and they range in size from a few centimeters to more than a meter (Traub and Wisseman 1968). They can be explained by the observation that the larvae are rarely observed individually, but occur mostly as clusters, which wait on stems or leaves to attach to a host (Gentry et al. 1963).
The seasonal occurrence of scrub typhus varies with the climate in different countries. For example, on the Korean peninsula, the peak incidence is during October and November (Noh et al. 2013), whereas in southern China, the number of cases peaks first in the summer months of June and July, then second in September and October (Wei et al. 2014a). In contrast, in some areas of Taiwan, no seasonal variation in the number of cases is observed (Tsai and Yeh 2013). Early observations of L. deliense and L. akamushi in Malaysia showed that the larvae in their natural habitats were sensitive to changes in temperature and humidity (Gentry et al. 1963), thus seasonal variation in disease occurrence is linked to fluctuations in temperature and rainfall. Many studies have described increased numbers of chiggers during periods of increased rainfall (Traub and Wisseman 1974), therefore scrub typhus outbreaks are often associated with monsoon and rainy seasons (Gurung et al. 2013) or periods of heavy rain (Faa et al. 2003). In temperate regions, scrub typhus cases are correlated more with temperature variations than with rainfall (Van Peenen et al. 1976; Kuo et al. 2011). There is evidence that climate change may be affecting the temporal occurrence and number of scrub typhus cases in some areas. For example, in Pingtan Island in eastern China, the known vector of scrub typhus is L. deliense, which previously appeared in late May, resulting in cases of scrub typhus in the summer. Since 2000, several cases of the disease have been reported in the spring, with mites observed as early as March. This could be linked to a rise in the average March temperatures since 1997 (Cao et al. 2006). Increases in temperature, sunshine, and rainfall were also linked to an increase in scrub typhus incidence in some areas of northern and southern China (Li et al. 2014; Yang et al. 2014).
Traditionally, scrub typhus is considered to be a disease associated with agricultural activities in rural areas (Kuo et al. 2011). Seroprevalence and outbreak studies have identified various occupational and behavioral risk factors for exposure to O. . These include being a farmer (particularly on dry, cultivated land), working in vegetable fields, bundling waste straw, living at the edge of a village, sitting on grass while taking breaks, and having close contact with rats (Mathai et al. 2003; Kweon et al. 2009a; Vallée et al. 2010; Kuo et al. 2011; Lyu et al. 2013; Wei et al. 2014b; Hu et al. 2015). A higher prevalence of antibodies against O. has been observed in older people (>50 or 60 years) in several studies (Bang et al. 2008; Brown et al. 1978a; Kuo et al. 2011; Vallée et al. 2010; Zheng et al. 2015), probably reflecting increased opportunities for exposure over the course of their lifetime. Many studies have demonstrated an increased risk of exposure for females compared to males (Bang et al. 2008; Kweon et al. 2009b; Kuo et al. 2011; Noh et al. 2013; Zheng et al. 2015), which may be linked to differences in the working behaviors of men and women in some areas. In South Korea, for example, men tend to work in the rice fields, where they use tools in a standing position. Women are more likely to be employed in dry fields, where they work with bare hands, typically in a squatting position in which they are more likely to come into contact with infected mites (Kweon et al. 2009b). However, in some areas such as Japan, there is no significant difference in exposure risk between the two sexes and equal numbers of scrub typhus cases are seen in males and females. This is believed to reflect cultural differences between different countries in terms of work and clothes (Bang et al. 2008). Military personnel deployed in endemic areas also have a high risk of contracting scrub typhus, due to their exposure to vegetation harboring infected mites. Subsequent to the aforementioned cases observed during World War II, outbreaks occurred during the Vietnam conflict and numerous cases have been recorded during training exercises and humanitarian operations (Corwin et al. 1999; Bavaro et al. 2005; Likeman 2006).
There is evidence to suggest that the epidemiology of scrub typhus is changing. Certainly, the disease is becoming urbanized and can no longer be considered a problem limited to rural areas. In recent years, numerous cases and outbreaks have been recognized in urban patients (Strickman et al. 1994; Wei et al. 2014a, b; Sethi et al. 2014; Park et al. 2015). One such outbreak, associated with a city park in China resulted in four deaths (Wei et al. 2014b). The urbanization of scrub typhus can be attributed to three main factors. First, the incursion of expanding human populations into agricultural or previously uninhabited areas has resulted in deforestation and the clearing of land, creating more suitable habitats for the vector mite species (Strickman et al. 1994; Vallée et al. 2010). Second, infected chiggers have been found in central urban locations, possibly due to their spread from surrounding areas (Park et al. 2015). Third, changes in the behavior of urban residents have made their contact with trombiculid mites more likely. For example, the working week in Korea was reduced to 5 days in 2004, allowing urban workers more time for recreational activities such as golf and climbing, or for agricultural activities such as harvesting chestnuts (Kweon et al. 2009b). In rural areas, changes in land use may affect scrub typhus epidemiology. For example, following Taiwan’s admission to the World Trade Organization , its rice market was exposed to foreign competition, leading to the abandonment of many rice paddies and reduced plowing in agricultural regions. This resulted in overgrowth of secondary vegetation and an increase in rodents and chiggers in these areas, increasing the risk of scrub typhus transmission (Kuo et al. 2012).
The incidence of scrub typhus is increasing in many countries. In part this may be due to improvements in diagnostic testing and better awareness. However, it is clear that the disease has reemerged in areas where cases had not occurred for many years (Lewis et al. 2003; Khan et al. 2012; Sethi et al. 2014) and that it is newly emerging in regions where it had not been recognized previously (Zhang et al. 2010; Hu et al. 2015). For example, in Japan where much of the early scrub typhus research was carried out, the disease was apparently absent for a period of around 10 years prior to its reemergence in 1976, with a subsequent increase in case numbers and expansion of endemic areas (Ishikura et al. 1985). Particularly rapid increases in disease incidence have been seen in some countries in recent years (Kweon et al. 2009b; Li et al. 2013; Zhang et al. 2013). In South Korea there were almost four times more cases in 2013 compared with 2001 (Lee et al. 2015a) and in China, 12.8 times more cases were reported in 2014 compared with 2006 (Wu et al. 2016). It is important to understand the changing epidemiology of scrub typhus to inform public health policies in both traditional and newly recognized endemic areas.
Scrub Typhus, the Disease
Clinical Manifestations
Scrub typhus presents as a mild disease in some patients, whereas others suffer from a more severe illness, which may even result in death. The bite of the chigger responsible for the transmission of O. to humans is usually painless and goes unnoticed by the patient (Watt and Parola 2003). Person-to-person transmission of scrub typhus is rare but a few instances have been recorded. Routes of transmission include needle-stick injuries via the placenta from mother to baby and stem cell transfusion (Wang et al. 1992; Kang et al. 2010).
In the days following infection, a small, (2–3 mm), reddish lesion may develop at the bite site and swelling may occur in the adjacent lymph nodes (Hayashi 1920). However, normally the first sign of illness is the sudden onset of a fever accompanied by nonspecific symptoms such as chills, headache, coughing, myalgia, nausea, diarrhea, and vomiting (Corbett 1943; Lee et al. 2013). The incubation period of scrub typhus is typically 7–10 days but can vary between 6 and 21 days.
In some patients, the small lesion at the bite site becomes larger, undergoing necrosis at the center and developing a blackened crust. This larger lesion is known as an eschar and it resembles a cigarette burn (Watt and Parola 2003) (Fig. 16.7).
Fig. 16.7.
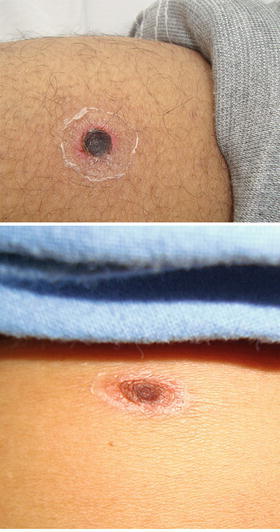
Examples of eschars on scrub typhus patients. Images courtesy of Munegowda Koralur; Kasturba Medical College, Manipal University, India
Eschars are usually found on parts of the body that tend to be warm and damp, such as the groin and axilla and often where pressure from clothing occurs, such as the waistband (Irons and Armstrong 1947). Differences in the distribution of eschars on males and females have been observed. In a study of 162 patients with eschars, the primary area for eschar development in males was within 30 cm below the umbilicus, whereas in females, the lesions were most prevalent on the front chest, above the umbilicus (Kim et al. 2007a). Another study demonstrated that preferential eschar development sites varied in different geographic areas (Zhang et al. 2012). Multiple eschars on a single scrub typhus patient are rare but have been documented (Kim et al. 2007a; Kaushik et al. 2014). Eschars are not present in all cases of scrub typhus and incidence rates are extremely variable. A low prevalence of 1–4 % has been recorded in several case series from India (Mathai et al. 2003; Sharma et al. 2005; Sethi et al. 2014), although another Indian study observed eschars in 45 % of the patients (Chrispal et al. 2010). In cohorts from other Asian countries, eschars have been present in a higher proportion (>60 %) of the patients (Sirisanthana et al. 2003; Kim et al. 2010; Hu et al. 2015). In addition to the eschar, some scrub typhus patients develop a maculopapular rash on the trunk, which appears 5–8 days after the onset of fever. The rash may extend to the arms and legs later in the infection (Irons and Armstrong 1947; Jeong et al. 2007). Other signs of the disease include generalized lymphadenopathy, acute hearing loss, conjunctival congestion, hepatomegaly, and splenomegaly (Noad and Haymaker 1953; Premaratna et al. 2006; Chrispal et al. 2010; Zhang et al. 2012).
In some patients, the clinical course of scrub typhus is more severe and a range of serious complications has been reported involving organs of the pulmonary, cardiac, abdominopelvic, and central nervous systems. These include interstitial pneumonia (Choi et al. 2000), acute respiratory distress syndrome (ARDS) (Park et al. 2000; Wang et al. 2007), myocarditis (Levine 1946), acute cholecystitis (Lee et al. 2015b), renal failure (Yen et al. 2003; Kim et al. 2010), acute kidney injury (Attur et al. 2013), gastrointestinal bleeding (Irons and Armstrong 1947), meningitis or meningoencephalitis (Kim et al. 2013), severe disseminated intravascular coagulation (Ono et al. 2012), and septic shock (Sethi et al. 2014). The diversity of these clinical manifestations is explained by the basic pathology of O. infection. The bacteria cause a focal or disseminated vasculitis due to the destruction of the endothelial cells (Choi et al. 2000; Chrispal et al. 2010). In cases of severe scrub typhus, it is not uncommon for multiple organs to be affected. For example, in a cohort of 116 patients with severe scrub typhus, 85 % experienced a dysfunction of three or more organ systems (Griffith et al. 2014). Risk factors that correlate with the development of severe disease include older age (>60 years), the absence of an eschar, low serum albumin (≤3.0 g/dL), elevated leukocytes (>10,000 μL), the presence of interstitial pneumonia, and elevated plasma inflammatory markers such as YKL-40 (Song et al. 2004; Chrispal et al. 2010; Kim et al. 2010; Otterdal et al. 2014). There is also evidence of a relationship between bacterial load and severity of the disease. A study of 155 Thai scrub typhus patients demonstrated a positive correlation between the concentration of O. in the blood taken on admission to hospital and the duration of illness, presence of an eschar, and hepatic enzyme levels. The bacterial load in patients that died was significantly higher than in those that recovered from their illness (Sonthayanon et al. 2009). A large systematic review of more than 19,000 untreated scrub typhus patients in 89 case series showed that overall, median mortality from the disease was only 6 %, although a wide range from 0 to 70 % was observed (Taylor et al. 2015). Variability may be due to numerous factors including the infecting strain, geographic area, and host factors (Taylor et al. 2015). Mortality rates in patients with severe complications are often higher. For example, studies of patients with ARDS have demonstrated mortality rates between 22 and 61 % (Wang et al. 2007; Chrispal et al. 2010; Griffith et al. 2014). Other predictors of mortality include the duration of fever, severity of illness on presentation to hospital, elevated serum creatinine (indicative of renal failure), and shock (Varghese et al. 2006; Chrispal et al. 2010; Griffith et al. 2014).
Coinfections of O. with several other pathogens have been reported. Diseases shown to occur concurrently with scrub typhus include pneumonia caused by (Lee et al. 2015c), murine typhus (Phommasone et al. 2013), Q fever (Lai et al. 2009), leptospirosis (Watt et al. 2003a; Chen et al. 2007; Sonthayanon et al. 2013), chicken pox (Chandramohan et al. 2015), malaria (Mahajan et al. 2014), and dengue (Kumar et al. 2014). Several of the coinfecting pathogens are susceptible to the antimicrobials used to treat scrub typhus. However, a coinfection should be suspected in scrub typhus patients if defervescence does not occur within 48–72 h of appropriate antimicrobial treatment and combination therapy may be required (Wei et al. 2012). Unusually, one O. tsutsugamushi coinfection may be advantageous to the patient. In Thailand, researchers observed that HIV-1 patients with scrub typhus experienced a reduction in viral load, and serum from an HIV-1 negative scrub typhus patient had a potent suppressive effect on the virus in vitro (Watt et al. 2000a). Another study was not able to replicate these results in vitro and showed that instead, HIV-1 replication was induced by O. (Moriuchi et al. 2003). Further work by the Thai researchers demonstrated that antibodies produced in acute scrub typhus suppressed CXCR4-HIV-1 viruses but not those utilizing the CCR5 coreceptor. However, the suppressive effect on the CXCR4-utilizing viruses was potent and long lasting (Watt et al. 2013). This was the first description of antibodies to one organism having a toxic effect on another infectious agent.
Diagnosis
The clinical presentation of scrub typhus is notoriously nonspecific. Since the primary manifestation is an acute, undifferentiated fever, when a patient first presents to a healthcare facility there is little to distinguish scrub typhus from other diseases such as typhoid, leptospirosis, and dengue, which are usually endemic in the same areas (Suttinont et al. 2006; Watt et al. 2003b). A recent Korean study developed a prediction rule for identifying suspected cases of scrub typhus in patients with acute undifferentiated fever. The rule comprised five predictors of disease that were derived from a multiple regression model, with each assigned a points value. Predictors were age ≥65 years old (two points), recent history of fieldwork or outdoor activity (one point), onset during the known outbreak period (September to December in Korea; two points), myalgia (one point), and presence of an eschar (two points). Using a cutoff value of four or more points, the sensitivity and specificity of the prediction rule for diagnosis of scrub typhus were 92.7 % and 90.9 %, respectively. While application of this prediction rule does not replace confirmatory testing, it can be used at the time of admission, allowing for the prompt initiation of treatment. However, it should not be used in more complicated cases of scrub typhus with clinical manifestations such as pneumonia or meningitis, and its use outside of Korea has not yet been validated (Jung et al. 2015).
The laboratory and radiographical findings in scrub typhus patients (Table 16.3 and Fig. 16.8) do not provide a definitive diagnosis, although in combination with other symptoms and signs they may lead to a strong suspicion of the disease. For example, in a cohort of Indian patients, a combination of elevated transaminases, thrombocytopenia, and leukocytosis had the best predictive values for diagnosing patients with acute undifferentiated fever (Varghese et al. 2006).
Table 16.3.
Summary of laboratory and radiographical findings in scrub typhus patients
| Investigation | Finding | Reported prevalence | Reference |
|---|---|---|---|
| White blood cell count |
Leukocytosis Leukocytes >10,000/μL |
9–54 % | Ogawa et al. (2002); Mathai et al. (2003); Song et al. (2004); Chrispal et al. (2010); Hu et al. (2015) |
| Platelet count |
Thrombocytopenia Platelets <10,000/μL |
21–44 % | Tsay and Chang (1998); Mathai et al. (2003); Song et al. (2004); Chrispal et al. (2010); Griffith et al. (2014); Hu et al. (2015) |
|
Severe thrombocytopenia Platelets < 50,000/μL |
25–47 % | ||
| Serum albumin |
Hypoalbuminemia <3.0 g/dL |
16–69 % | Song et al. (2004); Kim et al. (2010) |
| Serum myoglobin | Elevated | 35 % | Choi et al. (2000) |
| Renal function |
Elevated serum creatinine > 1.6 mg/dL or > 120 μmol/L |
15–37 % | Mathai et al. (2003); Song et al. (2004) |
|
Elevated blood urea nitrogen > 20 mg/dL |
49 % | Jung et al. (2015) | |
| Hepatic function |
Elevated transaminases Aspartate transaminase > 60 IU/L Alanine transaminase > 60 IU/L |
75–95 % | Chrispal et al. (2010), Song et al. (2004), Ogawa et al. (2002), Mathai et al. (2003), Choi et al. (2000), Zhang et al. (2012), Tsay and Chang (1998) |
|
Elevated C-reactive protein > 10 mg/dL |
25–96 % | Ogawa et al. (2002); Kim et al. (2010); Zhang et al. (2012); Hu et al. (2015) | |
| Elevated lactate dehydrogenase | 92 % | Ogawa et al. (2002); Kim et al. (2010) | |
|
Elevated bilirubin > 1 mg/dL or > 25 μmol/L |
18–29 % | Ogawa et al. (2002); Mathai et al. (2003); Kim et al. (2010); Jung et al. (2015) | |
| Partial oxygen pressure of arterial blood (PaO2) |
Hypoxia PaO2 < 60 mmHg |
24–34 % | Song et al. (2004); Chrispal et al. (2010) |
| Blood pressure |
Hypotension Arterial systolic pressure < 90 mmHg |
17 % | Song et al. (2004) |
| Chest x-ray | Normal | 50 % | Choi et al. (2000); Mathai et al. (2003); Song et al. (2004); Jeong et al. (2007) |
| Pulmonary abnormalities | 37–78 % | ||
| Pleural effusion | 42–55 % | ||
| Hilar enlargement | 14–45 % | ||
| Cardiomegaly | 15–38 % | ||
| Interstitial pneumonia | 51 % | Song et al. (2004) |
Fig. 16.8.

Chest X-ray of a scrub typhus patient. Radiograph shows a diffuse interstitial pneumonia. The patient developed ARDS but responded well to treatment with doxycycline. Image courtesy of Munegowda Koralur
Diagnostic tests for scrub typhus are mostly based on serological methodologies.
The oldest test, the Weil–Felix OXK, is based on the cross-reaction of the OXK antigen from Proteus mirabilis with scrub typhus IgM antibodies, resulting in agglutination of the serum (Amano et al. 1992). It has long been recognized that its sensitivity and specificity are poor, particularly in the early stages of O. infection (Brown et al. 1983; Isaac et al. 2004). For example, in a comparison between the Weil–Felix OXK and indirect immunoperoxidase (IIP) tests, only 10 % of serum samples positive by IIP gave a positive reaction in the Weil–Felix test within the first 9 days of illness (Amano et al. 1992). However, the Weil–Felix test is very cheap and easy to perform, therefore despite its severe limitations, it is still used in some resource-poor regions in which other serological tests are not available. It can be a useful tool in these settings, provided clinicians are aware of its pitfalls and results are interpreted in the correct clinical context (Mahajan et al. 2006).
The currently recognized gold standard test is the indirect immunofluorescence assay (IFA) (Blacksell et al. 2007), in which antibodies to O. present in the serum of the patient are bound to antigen on a slide, then detected using a fluorescently labeled anti-human antibody (Fig. 16.9).
Fig. 16.9.
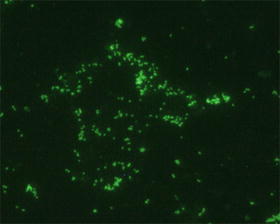
Indirect immunofluorescence assay for scrub typhus diagnosis. Image shows a positive result caused by the binding of antibodies to O. in the patient serum to antigen from the organism immobilized on the slide. A secondary anti-human antibody labeled with fluorescein isothiocyanate (FITC) enables detection of this reaction by fluorescent microscopy
The most commonly used antigens are a mixture of the Karp, Kato, and Gilliam serotypes (Blacksell et al. 2007), although in some areas, local serotypes are also included (Blacksell et al. 2007; Koh et al. 2010). A positive result is conventionally defined by an IgM titer of ≥1:400 on a single sample taken on admission, or by a fourfold (or greater) rise in titer between paired acute and convalescent samples to ≥1:200 (Brown et al. 1983). However, these conventional cutoff titers may lead to false-positive results, particularly where testing is performed on the admission sample alone (Blacksell et al. 2007; Lim et al. 2015a). A more recent study proposed revised cutoffs of ≥1:3200 for a single sample, or at least a fourfold rise to ≥1:3200 in the convalescent sample of a pair. These newly proposed optimal cutoffs resulted in an assay sensitivity and specificity of 81 % and 100 %, respectively (Lim et al. 2015a). It is recommended that if IFA is to be used for scrub typhus diagnosis in an endemic country, a local cutoff should be determined based on the scrub typhus seroprevalence rate in the healthy population. This better enables acute infection to be distinguished from previous exposure, which is especially important if testing is performed on only the acute sample (Blacksell et al. 2007). Besides difficulties with determining the appropriate cutoff, the IFA has additional limitations. Since a microscopist reading the slide determines the end-point titer, the test is inherently subjective. Inter- and intraoperator variability has been demonstrated and microscopists must undergo several months of training or be supervised by a more experienced staff member before their results can be considered reliable (Phetsouvanh et al. 2013). The main drawback of the IFA, however, is the relatively high cost and its requirement for a fluorescent microscope. This restricts its use in many of the resource-limited areas in which scrub typhus occurs (Koh et al. 2010). An alternative to the IFA is the IIP, in which the secondary antibody is labeled with peroxidase instead of a fluorochrome (Yamamoto and Minamishima 1982; Kelly et al. 1988). This eliminates the need for a fluorescent microscope, although the method suffers from the same limitations as IFA in terms of cutoff determination.
A scrub typhus IgM ELISA was first developed in 1979, when it was shown to have a similar sensitivity and specificity to the IFA (Dasch et al. 1979). An assay utilizing the O. tsutsugamushi-specific recombinant 56-kDa antigen is now available as a commercial kit and more recent studies have demonstrated a similar performance, with sensitivities in the range of 85–93 % and specificities between 94 and 97.5 % (Coleman et al. 2002; Prakash et al. 2006; Koraluru et al. 2015). Although the ELISA does not require specific training or equipment, the cost of the kit may still be too high for some laboratories and its availability is limited in some scrub typhus endemic countries (Isaac et al. 2004).
Numerous assays for the molecular detection of O. by PCR have been published and used in clinical settings. The most common targets of these assays are the genes encoding the 56-kDa antigen (Furuya et al. 1993; Horinouchi et al. 1996) and 47-kDa surface antigen (Jiang et al. 2004; Singhsilarak et al. 2005), plus the 16S rRNA (Sonthayanon et al. 2006; Kim et al. 2006a, 2016) and groEL genes (Park et al. 2005; Paris et al. 2009). PCR is most useful for diagnosis in the early stage of scrub typhus, before antibodies are detectable by serological methods. During this stage of the disease, PCR has been demonstrated to be more sensitive than IFA. In a Korean study, a positive result was obtained by nested PCR on the buffy coat of 19 out of 22 scrub typhus patients that were all negative by IFA on admission (Kim et al. 2006a) and similar results were obtained in a Thai study (Manosroi et al. 2003). The sensitivity of PCR in these two small studies of 21 and 118 patients was reportedly high (82.2 and 90.5 %) (Manosroi et al. 2003; Kim et al. 2006a). However, in larger studies of more than 180 patients, the diagnostic sensitivities of PCRs targeting the 16S rRNA gene, 56-kDa antigen gene, and 47-kDa antigen gene were only 44.8 %, 29 %, and 28.6 %, respectively (Sonthayanon et al. 2006; Watthanaworawit et al. 2013). Conflicting results have also been obtained from studies in which various PCR assays were compared. One demonstrated a real-time PCR targeting the 47-kDa antigen gene to be more sensitive than nested and conventional assays with the same target (Kim et al. 2011a). However, a more recent study showed that a conventional assay targeting the 16S rRNA gene was the most sensitive compared with conventional PCRs targeting different genes, nested and real-time assays (Kim et al. 2016). PCR sensitivity is likely influenced by several factors. Due to the sequence variability between different O. serotypes, particularly within the 56-kDa antigen gene, primers may not anneal as efficiently to some strains and may not even detect new serotypes. Detection may be more successful if PCR is performed on DNA extracted from the buffy coat of the blood rather than the whole EDTA blood sample, as the bacterial load has been shown to be approximately tenfold higher in the cellular fraction (Paris et al. 2008). Antibiotics have been shown to reduce the sensitivity of PCR detection of O. tsutsugamushi in the blood to 10 % by the fourth day after initiation of treatment (Kim and Byun 2008). Therefore, the assay should ideally be performed prior to antibiotic administration or at least only within 3 days after treatment initiation. Alternatively, PCR can be performed on eschars (Lee et al. 2006; Kim et al. 2006b). Detection of bacterial DNA from an eschar is less affected by antibiotic treatment (Kim et al. 2006b), but this sample is not available from all scrub typhus patients. Similarly to the IFA, the major disadvantage of PCR as a diagnostic method is its requirement for expensive, specialized equipment and staff training, which is not available in many scrub typhus endemic areas.
Besides serological and molecular diagnostic techniques, lesser used methods include immunohistochemical staining of the eschar (Kim et al. 2008) and isolation of O. , either in cell culture (Luksameetanasan et al. 2007) or by inoculation into a mouse (Casleton et al. 1998). Since the latter must be performed in a BSL-3 laboratory, it is not attempted routinely. Growth of the organism is often slow; therefore, results are of little use to the patient and treating clinician. However, it is important to obtain isolates of the bacteria to identify new serotypes and to allow further studies to be performed.
The common theme in scrub typhus diagnostics is the lack of resources in many areas in which the disease occurs. For this reason, there has been a focus in recent years on developing rapid, point-of-care tests that could provide an early, accurate diagnosis in these resource-limited settings. An alternative method to traditional PCR for the molecular detection of O. is the loop-mediated isothermal PCR assay (LAMP) (Paris et al. 2008, 2011). This method utilizes six primers and can amplify from a few copies to 109 copies in less than an hour. Amplification takes place at a single temperature (65 °C) and a positive result is determined by naked eye inspection of the reaction tube to visualize turbidity or a pellet (Notomi et al. 2000), therefore the only equipment required is a water bath or heat block. In an acute disease setting, LAMP was shown to have a diagnostic sensitivity of 53 % and specificity of 94 % (Paris et al. 2011).
Several immunochromatographic tests (ICTs) have been developed to provide point-of-care serological testing for scrub typhus, and some of these are now commercially available. In recent studies, while the specificity of these tests ranged between 68 and 95 %, sensitivity was lower at only 23–68 % (Blacksell et al. 2010a, b, 2012; Watthanaworawit et al. 2015). Diagnostic sensitivity was improved by 10–20 % if an ICT was combined with LAMP (Paris et al. 2011; Blacksell et al. 2012), but further research is required before a point-of-care scrub typhus test can be widely implemented. Most recently, publications have described a dot-ELISA (Rodkvamtook et al. 2015) and another ICT (Kingston et al. 2015), both based on recombinant 56-kDa antigens. Previously, a panel of scrub typhus infection criteria (STIC) was proposed for confirmation of positive cases, which could then be used in the evaluation of new diagnostic methods (Paris et al. 2011). A sample had to satisfy one of the four criteria, which were as follows: (1) positive cell culture isolation of O. ; (2) an admission IgM titer ≥ 1:12,800; (3) a fourfold rising IgM titer in paired serum samples; and (4) a positive result in at least two out of three PCR assays. Using STIC as a reference standard assumes that it is 100 % specific and sensitive, when in fact this may not be the case. A recent reanalysis of data from 161 patients using Bayesian latent class models gave different results for the sensitivities and specificities of various diagnostic tests compared to when STIC was used as the reference standard (Lim et al. 2015b). It is now recommended that any new tests for scrub typhus diagnosis be evaluated against a carefully selected set of existing tests using appropriate statistical models (Lim et al. 2015b).
Treatment and Control
Scrub typhus is readily curable using timely, appropriate antibiotics. Chloramphenicol was the first antibiotic shown to be effective at treating the disease (Smadel et al. 1949), but it was subsequently shown that fever and other symptoms were eliminated more rapidly using tetracycline (Sheehy et al. 1973). The mainstay of scrub typhus treatment is now doxycycline. Defervescence in the patient occurs so promptly (24–36 h) after initial administration of the drug that in some areas with limited access to diagnostic testing, this response to treatment is considered diagnostic for scrub typhus (Watt et al. 1996). The reduction in fever correlates with a reduction in production of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, which are up-regulated in scrub typhus patients (Chung et al. 2008). While short treatment regimens of a single dose (Brown et al. 1978b) or 3 days of doxycycline have been demonstrated to be effective (Song et al. 1995), caution should be exhibited if this treatment is initiated within the first 5 days of disease onset. Since the antibiotics shown to be effective against O. are bacteriostatic rather than bactericidal, they slow the bacterial growth while the patient mounts an immune response to the infection. Thus, there have been cases of patients relapsing when treatment was terminated too early, before the development of antibodies (Smadel et al. 1950; Olson et al. 1981; Im et al. 2014). Doxycycline failure was also observed in a patient who was administered antacids for the gastrointestinal symptoms of scrub typhus in addition to the antibiotic. The patient developed scrub typhus meningoencephalitis during the course of the treatment, believed to have been caused by the antacids reducing the doxycycline concentration in the serum to subtherapeutic levels (Kim et al. 2011b). Delayed fever resolution in some patients treated with doxycycline in northern Thailand led to the discovery of O. strains that were resistant to both doxycycline and chloramphenicol (Watt et al. 1996). The driver for this resistance is unclear, although it was postulated that antibiotics may have reached the chigger reservoir via rats feeding on animal food (Rosenberg 1997).
Alternative antibiotics for the treatment of scrub typhus include rifampin (Im et al. 2014; Watt et al. 2000b) and the macrolides azithromycin, telithromycin (Kim et al. 2007b), and roxithromycin (Lee et al. 2003). Rifampin was shown to reduce fever more rapidly than doxycycline (Watt et al. 2000b), although its efficacy in cases of severe scrub typhus has not been evaluated. Azithromycin has been shown to be as effective as doxycycline for treatment of both mild to moderate and severe scrub typhus (Kim et al. 2004b; Jang et al. 2014). Its use is recommended for the treatment of scrub typhus in pregnant women and children under 8 years old, as doxycycline use in these groups is contraindicated due to association of the drug with fetal risk and permanent discoloration of the teeth (Kim et al. 2006c; Cross et al. 2015). However, evidence for these risks was based on tetracycline rather than the newer, semisynthetic doxycycline and there are calls for the contraindication of doxycycline to be reevaluated (Cross et al. 2015). Conflicting reports are available regarding the efficacy of the fluoroquinolones against O. . One study showed that levofloxacin was an effective treatment for scrub typhus, although patients took longer to become afebrile compared to those administered tetracycline antibiotics (Tsai et al. 2010). Other studies showed that O. tsutsugamushi strains were resistant to ciprofloxacin and ofloxacin, and demonstrated the presence of a mutation (Ser83Leu) associated with resistance in the quinolone resistance-determining region of the gyrA gene (Tantibhedhyangkul et al. 2010; Jang et al. 2013). Therefore, the use of fluoroquinolones is not advocated.
In the case of coinfections with O. and another pathogen, combination therapy may be required for the effective resolution of both diseases. For example, the recommended treatment for a patient with concurrent scrub typhus and leptospirosis is ceftriaxone and doxycycline (Lee et al. 2014). In cases of severe scrub typhus pneumonia and ARDS, empirical treatment with a beta-lactam antibiotic with doxycycline or a macrolide is sometimes used (Lee et al. 2014). This practice may need to be revised in light of the finding that the efficacies of doxycycline, azithromycin, and rifampin against O. are lessened in the presence of the beta-lactam cefotaxime (Lee et al. 2014). In contrast, the antimalarial agent chloroquine has been shown to increase the antimicrobial effect of doxycycline against O. tsutsugamushi in vitro (Son and Chung 2014).
Although most patients make a full recovery from scrub typhus, there is evidence that O. tsutsugamushi is not completely cleared and may persist in the body. In an early study, the organism was isolated from an asymptomatic patient who had confirmed scrub typhus infection more than 1 year prior (Smadel et al. 1952). More recently, O. was isolated from six recovered patients, up to 18 months after disease onset (Chung et al. 2012). The significance of persistent infection is not known but it is possible that recrudescence may occur after primary infection (Im et al. 2014).
Despite extensive research, there is currently no vaccine available to prevent scrub typhus. Chemoprophylaxis of a 200 mg weekly dose of doxycycline has been demonstrated to be effective at preventing the disease and is recommended for people entering a scrub typhus endemic region where they are likely to have a high risk of exposure to mites (Olson et al. 1980; Twartz et al. 1982). Generally however, control of scrub typhus relies on measures to reduce the risk of chigger bites. Toward the end of World War II, military personnel in scrub typhus endemic areas were encouraged to treat their uniforms with the mosquito repellent dimethylphthalate, which was also effective against chiggers. The Australian Army introduced dibutylphthalate to its soldiers as “ antimite fluid,” which was more resistant to washing (Philip 1948; Frances 2011), and a 90 % reduction in scrub typhus cases was achieved through its use (Mcculloch 1946). It is now recommended that military uniforms be treated with permethrin (Frances et al. 1992; Frances 2011) or dibutylphthalate (Hengbin et al. 2006) to reduce chigger (and other arthropod) attachment.
Local health authorities in endemic areas have implemented education programs and proposed recommendations to civilian populations in order to reduce cases of scrub typhus. Preventative measures include wearing protective clothing such as arm warmers or gumboots during work activities, washing after work, changing into a different set of clothes to sleep, and using insect repellent (Sharma et al. 2009; Kim et al. 2012). The commonly used insect repellent, DEET (N-N-diethyl-m-toluamide), is effective against chiggers (Buescher et al. 1984) and recent studies have shown some essential oils from plant species, particularly (clove) and Melaleuca alternifolia (tea tree) have a chigger repellent effect. These may be cheaper and less harmful than synthetic chemicals (Eamsobhana et al. 2009; Rodkvamtook et al. 2012). A scrub typhus prevention program implemented in several public health centers in Korea was attributed to a decline in the reported cases of disease. A cost–benefit analysis estimated it saved $6.66 million each year by reducing medical costs and productivity losses (Kim et al. 2012), therefore similar programs may be beneficial in other endemic areas. In addition to personal protective measures, steps can be taken to control the environment and rodent populations, making conditions less favorable for mites. These include the trapping or poisoning of rodents, regular cleaning of buildings inside and out, management of human and food waste, and maintenance of the natural habitat such as preventing weed growth around buildings (Hengbin et al. 2006).
Conclusions
Scrub typhus caused by O. remains a serious and potentially life-threatening disease. Despite years of research, there are still many unresolved issues relating to disease ecology, epidemiology, pathogenesis, diagnosis, and immunity (Paris et al. 2013). Work continues to address these problems and increase our understanding of the disease and its causative agent.
Acknowledgements
Thanks are due to Dr. Stephen Graves and Dr. John Stenos from the Australian Rickettsial Reference Laboratory for their intellectual input.
Contributor Information
Sunil Thomas, Phone: +11646-331-8232, Email: suntom2@gmail.com.
Gemma Vincent, Email: gvince@barwonhealth.org.au.
References
- Amano K, Suzuki N, Hatakeyama H, Kasahara Y, Fujii S, et al. The reactivity between rickettsiae and Weil-Felix test antigens against sera of rickettsial disease patients. Acta Virol. 1992;36:67. [PubMed] [Google Scholar]
- Amano K, Tamura A, Ohashi N, Urakami H, Kaya S, et al. Deficiency of peptidoglycan and lipopolysaccharide components in Rickettsia tsutsugamushi. Infect and Immun. 1987;55:2290–2292. doi: 10.1128/iai.55.9.2290-2292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SGE, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. doi: 10.1016/S0966-842X(98)01312-2. [DOI] [PubMed] [Google Scholar]
- Arai S, Tabara K, Yamamoto N, Fujita H, Itagaki A, et al. Molecular phylogenetic analysis of Orientia tsutsugamushi based on the groES and groEL genes. Vector Borne Zoonot Dis. 2013;13:825–9. doi: 10.1089/vbz.2012.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn PM, Craig CF. A comparative study of tsutsugamushi disease and spotted or tick fever of Montana. Philippine J Sci. 1908;3:1–7. [Google Scholar]
- Attur RP, Kuppasamy S, Bairy M, Nagaraju SP, Pammidi NR, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17:725–9. doi: 10.1007/s10157-012-0753-9. [DOI] [PubMed] [Google Scholar]
- Balcells ME, Rabagliati R, Garcia P, Poggi H, Oddo D, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659–63. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang HA, Lee MJ, Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61:148–50. [PubMed] [Google Scholar]
- Bavaro MF, Kelly DJ, Dasch GA, Hale BR, Olson P. History of U.S. military contributions to the study of rickettsial diseases. Mil Med. 2005;170:49–60. doi: 10.7205/MILMED.170.4S.49. [DOI] [PubMed] [Google Scholar]
- Bell EJ, Bennett BL, Whitman L. Antigenic differences between strains of scrub typhus as demonstrated by cross-neutralization tests. Proc Soc Exp Biol Med. 1946;62:134–7. doi: 10.3181/00379727-62-15398. [DOI] [PubMed] [Google Scholar]
- Bengston IA. Apparent serological heterogeneity among strains of tsutsugamushi disease. Pub Health Rep. 1945;60:1483–1488. doi: 10.2307/4585496. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Smadel JE, Gauld RL. Differences in strains of Rickettsia orientalis as demonstrated by cross-neutralization tests. J Bacteriol. 1947;54:93. [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2009;37:D26–D31. doi: 10.1093/nar/gkn723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer AR, Viebrock L, Rodino KG, Miller DP, Tegels BK, et al. Orientia tsutsugamushi strain Ikeda ankyrin repeat-containing proteins recruit SCF1 ubiquitin ligase machinery via poxvirus-like F-box motifs. J Bacteriol. 2015;197:3097–109. doi: 10.1128/JB.00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, et al. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]
- Blacksell SD, Jenjaroen K, Phetsouvanh R, Tanganuchitcharnchai A, Phouminh P, et al. Accuracy of rapid IgM-based immunochromatographic and immunoblot assays for diagnosis of acute scrub typhus and murine typhus infections in Laos. Am J Trop Med Hyg. 2010;83:365–9. doi: 10.4269/ajtmh.2010.09-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacksell SD, Jenjaroen K, Phetsouvanh R, Wuthiekanun V, Day NP, et al. Accuracy of AccessBio immunoglobulin M and total antibody rapid immunochromatographic assays for the diagnosis of acute scrub typhus infection. Clin Vaccine Immunol. 2010;17:263–6. doi: 10.1128/CVI.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacksell SD, Paris DH, Chierakul W, Wuthiekanun V, Teeratakul A, et al. Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin Vaccine Immunol. 2012;19:391–5. doi: 10.1128/CVI.05478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeman FM, Elisberg BL. Studies of the antibody response in scrub typhus employing indirect immunofluorescence. Acta Med Biol (Niigata) 1967;15:105–11. [PubMed] [Google Scholar]
- Bozeman FM, Hopps HE, Danauskas JX, Jackson EB, Smadel JE. Study on the growth of Rickettsiae. I. A tissue culture system for quantitative estimations of Rickettsia tsutsugamushi. J Immunol. 1956;76:475–88. [PubMed] [Google Scholar]
- Brown GW, Robinson DM, Huxsoll DL. Serological evidence for a high incidence of transmission of Rickettsia tsutsugamushi in two Orang Asli settlements in peninsular Malaysia. Am J Trop Med Hyg. 1978;27:121–124. doi: 10.4269/ajtmh.1978.27.121. [DOI] [PubMed] [Google Scholar]
- Brown GW, Saunders JP, Singh S, Huxsoll DL, Shirai A. Single dose doxycycline therapy for scrub typhus. Trans R Soc Trop Med Hyg. 1978;72:412–416. doi: 10.1016/0035-9203(78)90138-4. [DOI] [PubMed] [Google Scholar]
- Brown GW, Shirai A, Rogers C, Groves MG. Diagnostic criteria for scrub typhus: probability values for immunofluorescent antibody and Proteus OXK agglutinin titers. Am J Trop Med Hyg. 1983;32:1101–7. doi: 10.4269/ajtmh.1983.32.1101. [DOI] [PubMed] [Google Scholar]
- Buescher MD, Rutledge LC, Wirtz RA, Nelson JH, Inase JL. Repellent tests against Leptotrombidium (Leptotrombidium) fletcheri (Acari: Trombiculidae) J Med Entomol. 1984;21:278–82. doi: 10.1093/jmedent/21.3.278. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Varma MGR. Trans-stadial and transovarial development of disease agents in arthropods. Annual Rev of Entomol. 1967;12:347–376. doi: 10.1146/annurev.en.12.010167.002023. [DOI] [PubMed] [Google Scholar]
- Cadigan FC, Andre RG, Bolton M, Gan E, Walker JS. The effect of habitat on the prevalence of human scrub typhus in Malaysia. Trans R Soc Trop Med Hyg. 1972;66:582–587. doi: 10.1016/0035-9203(72)90303-3. [DOI] [PubMed] [Google Scholar]
- Cao M, Guo H, Tang T, Wang C, Li X, et al. Spring scrub typhus, People's Republic of China. Emerg Infect Dis. 2006;12:1463–5. doi: 10.3201/eid1209.060257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casleton BG, Salata K, Dasch GA, Strickman D, Kelly DJ. Recovery and viability of Orientia tsutsugamushi from packed red cells and the danger of acquiring scrub typhus from blood transfusion. Transfusion. 1998;38:680–9. doi: 10.1046/j.1537-2995.1998.38798346638.x. [DOI] [PubMed] [Google Scholar]
- Chandramohan A, Venkatesh S, Dhandapany G, Stephen S. Scrub typhus co-infection in an adolescent girl with varicella. Indian Pediatr. 2015;52:891–2. doi: 10.1007/s13312-015-0739-2. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kang JS, Lee WK, Choi MS, Lee JH. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J Clin Microbiol. 1990;28:685–688. doi: 10.1128/jcm.28.4.685-688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YS, Cheng SL, Wang HC, Yang PC. Successful treatment of pulmonary hemorrhage associated with leptospirosis and scrub typhus coinfection by early plasma exchange. J Formos Med Assoc. 2007;106:S1–6. doi: 10.1016/S0929-6646(09)60344-2. [DOI] [PubMed] [Google Scholar]
- Cho BA, Cho NH, Seong SY, Choi MS, Kim IS. Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun. 2010;78:1915–23. doi: 10.1128/IAI.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NH, Kim HR, Lee JH, Kim SY, Kim J, et al. The Orientia tsutsugamushi genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc Natl Acad Sci U S A. 2007;104:7981–6. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Cheong TC, Ha NY, Ko Y, Cho CH, et al. Orientia tsutsugamushi subverts dendritic cell functions by escaping from autophagy and impairing their migration. PLoS Negl Trop Dis. 2013;7:e1981. doi: 10.1371/journal.pntd.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Kim SJ, Lee JY, Pai HJ, Lee KY, et al. Scrub typhus: radiological and clinical findings. Clin Radiol. 2000;55:140–4. doi: 10.1053/crad.1999.0336. [DOI] [PubMed] [Google Scholar]
- Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, et al. Scrub typhus: an unrecognized threat in South India - clinical profile and predictors of mortality. Trop Doct. 2010;40:129–33. doi: 10.1258/td.2010.090452. [DOI] [PubMed] [Google Scholar]
- Chu H, Lee JH, Han SH, Kim SY, Cho NH, et al. Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes. Infect Immun. 2006;74:4246–53. doi: 10.1128/IAI.01620-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DR, Lee YS, Lee SS. Kinetics of inflammatory cytokines in patients with scrub typhus receiving doxycycline treatment. J Infect. 2008;56:44–50. doi: 10.1016/j.jinf.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Chung MH, Lee JS, Baek JH, Kim M, Kang JS. Persistence of Orientia tsutsugamushi in humans. J Korean Med Sci. 2012;27:231–5. doi: 10.3346/jkms.2012.27.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CF, Cox HR. The cultivation of Rickettsia orientalis in fertile hens' eggs. J Bacteriol. 1946;51:629. [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci USA. 2008;105:9379–84. doi: 10.1073/pnas.0712241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503. doi: 10.4269/ajtmh.2002.67.497. [DOI] [PubMed] [Google Scholar]
- Corbett AJ. Scrub typhus. Army Med Bull. 1943;70:34–54. [Google Scholar]
- Corwin A, Soderquist R, Suwanabun N, Sattabongkot J, Martin L, et al. Scrub typhus and military operations in Indochina. Clin Infect Dis. 1999;29:940–941. doi: 10.1086/520468. [DOI] [PubMed] [Google Scholar]
- Cosson JF, Galan M, Bard E, Razzauti M, Bernard M, et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasit Vectors. 2015;8:172. doi: 10.1186/s13071-015-0784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross R, Ling C, Day NP, Mcgready R, Paris DH. Revisiting doxycycline in pregnancy and early childhood - time to rebuild its reputation? Expert Opin Drug Saf. 2016;15:367–82. doi: 10.1517/14740338.2016.1133584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby AC, Cho N-H, Fuxelius H-H, Westberg J, Andersson SGE. Intracellular pathogens go extreme: genome evolution in the Rickettsiales. Trends Genet. 2007;23:511–520. doi: 10.1016/j.tig.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Dasch GA, Halle S, Bourgeois AL. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J Clin Microbiol. 1979;9:38–48. doi: 10.1128/jcm.9.1.38-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamsobhana P, Yoolek A, Kongkaew W, Lerdthusnee K, Khlaimanee N, et al. Laboratory evaluation of aromatic essential oils from thirteen plant species as candidate repellents against Leptotrombidium chiggers (Acari: Trombiculidae), the vector of scrub typhus. Exp Appl Acarol. 2009;47:257–62. doi: 10.1007/s10493-008-9214-2. [DOI] [PubMed] [Google Scholar]
- Eisemann CS, Osterman JV. Identification of strain-specific and group-reactive antigenic determinants on the Karp, Gilliam and Kato strains of Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1985;34:1173–1178. doi: 10.4269/ajtmh.1985.34.1173. [DOI] [PubMed] [Google Scholar]
- Elisberg BL, Campbell JM, Bozeman FM. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12:18–25. [PubMed] [Google Scholar]
- Enatsu T, Urakami H, Tamura A. Phylogenetic analysis of Orientia tsutsugamushi strains based on the sequence homologies of 56-kDa type-specific antigen genes. FEMS Microbiol Lett. 1999;180:163–9. doi: 10.1111/j.1574-6968.1999.tb08791.x. [DOI] [PubMed] [Google Scholar]
- Ewing EP, Jr, Takeuchi A, Shirai A, Osterman JV. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978;19:1068–75. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faa AG, Mcbride WJ, Garstone G, Thompson RE, Holt P. Scrub typhus in the Torres Strait islands of north Queensland, Australia. Emerg Infect Dis. 2003;9:480–2. doi: 10.3201/eid0904.020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MY, Walker DH, Yu SR, Liu QH. Epidemiology and ecology of rickettsial diseases in the People's Republic of China. Rev Infect Dis. 1987;9:823–40. doi: 10.1093/clinids/9.4.823. [DOI] [PubMed] [Google Scholar]
- Frances SP. Rickettsial diseases of military importance: An Australian perspective. J Mil Vet Health. 2011;19:27–30. [Google Scholar]
- Frances SP, Watcharapichat P, Phulsuksombati D. Vertical transmission of Orientia tsutsugamushi in two lines of naturally infected Leptotrombidium deliense (Acari: Trombiculidae) J Med Entomol. 2001;38:17–21. doi: 10.1603/0022-2585-38.1.17. [DOI] [PubMed] [Google Scholar]
- Frances SP, Watcharapichat P, Phulsuksombati D, Tanskul P. Transmission of Orientia tsutsugamushi, the aetiological agent for scrub typhus, to co-feeding mites. Parasitology. 2000;120(Pt 6):601–7. doi: 10.1017/S0031182099005909. [DOI] [PubMed] [Google Scholar]
- Frances SP, Yeo AE, Brooke EW, Sweeney AW. Clothing impregnations of dibutylphthalate and permethrin as protectants against a chigger mite, Eutrombicula hirsti (Acari: Trombiculidae) J Med Entomol. 1992;29:907–10. doi: 10.1093/jmedent/29.6.907. [DOI] [PubMed] [Google Scholar]
- Furuya Y, Yoshida Y, Katayama T, Yamamoto S, Kawamura A., Jr Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31:1637–40. doi: 10.1128/jcm.31.6.1637-1640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Subversion of host cell signaling by Orientia tsutsugamushi. Microbes Infect. 2011;13:638–48. doi: 10.1016/j.micinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Gentry JW, Yueh CS, Wah PO. Preliminary observations on Leptotrombidium (Leptotrombidium) akamushi and Leptotrombidium (Leptotrombidium) deliensis in their natural habitat in Malaya: (Acarina: Trombiculidae) Am J Hyg. 1963;78:181–90. doi: 10.1093/oxfordjournals.aje.a120337. [DOI] [PubMed] [Google Scholar]
- Georgiades K, Merhej V, El Karkouri K, Raoult D, Pontarotti P. Gene gain and loss events in Rickettsia and Orientia species. Biology Direct. 2011;6:6. doi: 10.1186/1745-6150-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani RP, Ghorbani AJ, Jain MK, Walker DH. A case of scrub typhus probably acquired in Africa. Clin Infect Dis. 1997;25:1473–4. doi: 10.1086/516990. [DOI] [PubMed] [Google Scholar]
- Giengkam Suparat, Blakes Alex, Utsahajit Peemdej, Chaemchuen Suwittra, Atwal Sharanjeet, Blacksell Stuart D., Paris Daniel H., Day Nicholas P. J., Salje Jeanne. Improved Quantification, Propagation, Purification and Storage of the Obligate Intracellular Human Pathogen Orientia tsutsugamushi. PLOS Neglected Tropical Diseases. 2015;9(8):e0004009. doi: 10.1371/journal.pntd.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez DF. Staining rickettsiae in yolk-sac cultures. Stain Technol. 1964;39:135–40. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Glazebrook JS, Campbell RS, Hutchinson GW, Stallman ND. Rodent zoonoses in North Queensland: the occurrence and distribution of zoonotic infections in North Queensland rodents. Aust J Exp Biol Med Sci. 1978;56:147–56. doi: 10.1038/icb.1978.16. [DOI] [PubMed] [Google Scholar]
- Griffith M, Peter JV, Karthik G, Ramakrishna K, Prakash JA, et al. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med. 2014;18:497–502. doi: 10.4103/0972-5229.138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung S, Pradhan J, Bhutia PY. Outbreak of scrub typhus in the North East Himalayan region-Sikkim: an emerging threat. Indian J Med Microbiol. 2013;31:72–4. doi: 10.4103/0255-0857.108729. [DOI] [PubMed] [Google Scholar]
- Ha NY, Cho NH, Kim YS, Choi MS, Kim IS. An autotransporter protein from Orientia tsutsugamushi mediates adherence to nonphagocytic host cells. Infect Immun. 2011;79:1718–27. doi: 10.1128/IAI.01239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. Factors influencing Rickettsia tsutsugamushi infection of cultured cells. Am J Trop Med Hyg. 1987;36:621–30. doi: 10.4269/ajtmh.1987.36.621. [DOI] [PubMed] [Google Scholar]
- Hayashi N. Etiology of tsutsugamushi disease. J Parasitol. 1920;7:53–68. doi: 10.2307/3270957. [DOI] [Google Scholar]
- Hengbin G, Min C, Kaihua T, Jiaqi T. The foci of scrub typhus and strategies of prevention in the Spring in Pingtan Island, Fujian Province. Ann N Y Acad Sci. 2006;1078:188–96. doi: 10.1196/annals.1374.130. [DOI] [PubMed] [Google Scholar]
- Horinouchi H, Murai K, Okayama A, Nagatomo Y, Tachibana N, et al. Genotypic identification of Rickettsia tsutsugamushi by restriction fragment length polymorphism analysis of DNA amplified by the polymerase chain reaction. Am J Trop Med Hyg. 1996;54:647–51. doi: 10.4269/ajtmh.1996.54.647. [DOI] [PubMed] [Google Scholar]
- Hu J, Tan Z, Ren D, Zhang X, He Y, et al. Clinical characteristics and risk factors of an outbreak with scrub typhus in previously unrecognized areas, Jiangsu Province, China 2013. PLoS One. 2015;10:e0125999. doi: 10.1371/journal.pone.0125999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im JH, Baek JH, Lee JS, Chung MH, Lee SM, et al. In vitro bacteriostatic effects of rifampin on Orientia tsutsugamushi. J Korean Med Sci. 2014;29:183–9. doi: 10.3346/jkms.2014.29.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons EM, Armstrong HE. Scrub typhus in Dutch New Guinea. Ann Intern Med. 1947;26:201–20. doi: 10.7326/0003-4819-26-2-201. [DOI] [PubMed] [Google Scholar]
- Isaac R., Varghese G. M., Mathai E., Manjula J., Joseph I. Scrub Typhus: Prevalence and Diagnostic Issues in Rural Southern India. Clinical Infectious Diseases. 2004;39(9):1395–1396. doi: 10.1086/424748. [DOI] [PubMed] [Google Scholar]
- Ishikura M, Watanabe M, Morita O, Uetake H. Epidemiological studies on the background of the endemic occurrence of tsutsugamushi disease in Toyama Prefecture. I. Epidemiology of infection with Rickettsia tsutsugamushi among field rodents in endemic and nonendemic areas. Microbiol Immunol. 1985;29:859–72. doi: 10.1111/j.1348-0421.1985.tb00888.x. [DOI] [PubMed] [Google Scholar]
- Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–9. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EB, Danauskas JX, Smadel JE, Fuller HS, Coale MC, et al. Occurrence of Rickettsia tsutsugamushi in Korean rodents and chiggers. Am J Hyg. 1957;66:309–20. doi: 10.1093/oxfordjournals.aje.a119904. [DOI] [PubMed] [Google Scholar]
- Jang HC, Choi SM, Jang MO, Ahn JH, Kim UJ, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667–71. doi: 10.3346/jkms.2013.28.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MO, Jang HC, Kim UJ, Ahn JH, Kang SJ, et al. Outcome of intravenous azithromycin therapy in patients with complicated scrub typhus compared with that of doxycycline therapy using propensity-matched analysis. Antimicrob Agents Chemother. 2014;58:1488–93. doi: 10.1128/AAC.01996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, et al. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics. 2007;27:161–72. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, et al. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–6. [PubMed] [Google Scholar]
- Jung HC, Chon SB, Oh WS, Lee DH, Lee HJ. Etiologies of acute undifferentiated fever and clinical prediction of scrub typhus in a non-tropical endemic area. Am J Trop Med Hyg. 2015;92:256–61. doi: 10.4269/ajtmh.14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosaka T, Kimura E. Electron microscopic observations of Orientia tsutsugamushi in salivary gland cells of naturally infected Leptotrombidium pallidum larvae during feeding. Microbiol Immunol. 2003;47:727–33. doi: 10.1111/j.1348-0421.2003.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Park KH, Jung SI, Jang HC, Ji SY, et al. Scrub typhus induced by peripheral blood stem cell transplantation in the immunocompromised patient: diagnostic usefulness of nested polymerase chain reaction. Transfusion. 2010;50:467–70. doi: 10.1111/j.1537-2995.2009.02442.x. [DOI] [PubMed] [Google Scholar]
- Kaushik RM, Kaushik R, Bhargava A. Multiple eschars in scrub typhus. Trop Med Health. 2014;42:65–6. doi: 10.2149/tmh.2013-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Hauptmann M, Kolbaum J, Gharaibeh M, Neumann M, et al. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl Trop Dis. 2014;8:e3064. doi: 10.1371/journal.pntd.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Fuerst PA, Ching W-M, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clinical Infectious Diseases. 2009;48:S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Wong PW, Gan E, Lewis GE., Jr Comparative evaluation of the indirect immunoperoxidase test for the serodiagnosis of rickettsial disease. Am J Trop Med Hyg. 1988;38:400–6. doi: 10.4269/ajtmh.1988.38.400. [DOI] [PubMed] [Google Scholar]
- Khan SA, Dutta P, Khan AM, Topno R, Borah J, et al. Re-emergence of scrub typhus in northeast India. Int J Infect Dis. 2012;16:e889–90. doi: 10.1016/j.ijid.2012.05.1030. [DOI] [PubMed] [Google Scholar]
- Kim CM, Cho MK, Kim DM, Yun NR, Kim SW, et al. Accuracy of conventional PCR targeting the 16S rRNA Gene with the Ot-16sRF1 and Ot-16sRR1 primers for diagnosis of scrub typhus: a case-control study. J Clin Microbiol. 2016;54:178–9. doi: 10.1128/JCM.01754-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Byun JN. Effects of antibiotic treatment on the results of nested PCRs for scrub typhus. J Clin Microbiol. 2008;46:3465–6. doi: 10.1128/JCM.00634-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Chung JH, Yun NR, Kim SW, Lee JY, et al. Scrub typhus meningitis or meningoencephalitis. Am J Trop Med Hyg. 2013;89:1206–11. doi: 10.4269/ajtmh.13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Kim HL, Park CY, Yang TY, Lee JH, et al. Clinical usefulness of eschar polymerase chain reaction for the diagnosis of scrub typhus: a prospective study. Clin Infect Dis. 2006;43:1296–300. doi: 10.1086/508464. [DOI] [PubMed] [Google Scholar]
- Kim DM, Kim SW, Choi S-H, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. 2010;10:108–108. doi: 10.1186/1471-2334-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Kim YS, Cho HY, Lee YB. Scrub typhus meningoencephalitis occurring during doxycycline therapy for Orientia tsutsugamushi. Diagn Microbiol Infect Dis. 2011;69:271–4. doi: 10.1016/j.diagmicrobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Kim DM, Park CJ, Lim SC, Park KH, Jang WJ, et al. Diagnosis of scrub typhus by immunohistochemical staining of Orientia tsutsugamushi in cutaneous lesions. Am J Clin Pathol. 2008;130:543–51. doi: 10.1309/X17HNNJKMYGHT4HP. [DOI] [PubMed] [Google Scholar]
- Kim DM, Park G, Kim HS, Lee JY, Neupane GP, et al. Comparison of conventional, nested, and real-time quantitative PCR for diagnosis of scrub typhus. J Clin Microbiol. 2011;49:607–12. doi: 10.1128/JCM.01216-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Won KJ, Park CY, Yu KD, Kim HS, et al. Distribution of eschars on the body of scrub typhus patients: a prospective study. Am J Trop Med Hyg. 2007;76:806–809. [PubMed] [Google Scholar]
- Kim DM, Yu KD, Lee JH, Kim HK, Lee SH. Controlled trial of a 5-day course of telithromycin versus doxycycline for treatment of mild to moderate scrub typhus. Antimicrob Agents Chemother. 2007;51:2011–5. doi: 10.1128/AAC.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DM, Yun NR, Yang TY, Lee JH, Yang JT, et al. Usefulness of nested PCR for the diagnosis of scrub typhus in clinical practice: A prospective study. Am J Trop Med Hyg. 2006;75:542–5. [PubMed] [Google Scholar]
- Kim HR, Choi MS, Kim IS. Role of syndecan-4 in the cellular invasion of Orientia tsutsugamushi. Microb Pathog. 2004;36:219–25. doi: 10.1016/j.micpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee E, Rhee HC. Cost-benefit analysis of the tsutsugamushi disease prevention program in South Korea. Jpn J Infect Dis. 2012;65:222–7. doi: 10.7883/yoken.65.222. [DOI] [PubMed] [Google Scholar]
- Kim MK, Kee SH, Cho KA, Chung MH, Lim BU, et al. Apoptosis of endothelial cell line ECV304 persistently infected with Orientia tsutsugamushi. Microbiol Immunol. 1999;43:751–7. doi: 10.1111/j.1348-0421.1999.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ihn KS, Han SH, Seong SY, Kim IS, et al. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect Immun. 2001;69:494–500. doi: 10.1128/IAI.69.1.494-500.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Lee HJ, Chang M, Son SK, Rhee YE, et al. Scrub typhus during pregnancy and its treatment: a case series and review of the literature. Am J Trop Med Hyg. 2006;75:955–9. [PubMed] [Google Scholar]
- Kim YS, Yun H-J, Shim SK, Koo SH, Kim SY, et al. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis. 2004;39:1329–1335. doi: 10.1086/425008. [DOI] [PubMed] [Google Scholar]
- Kingston HW, Blacksell SD, Tanganuchitcharnchai A, Laongnualpanich A, Basnyat B, et al. Comparative accuracy of the Inbios Scrub Typhus Detect IgM rapid test for the detection of IgM antibodies by using conventional serology. Clin Vaccine Immunol. 2015;22:1130–2. doi: 10.1128/CVI.00390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y, Choi JH, Ha NY, Kim IS, Cho NH, et al. Active escape of Orientia tsutsugamushi from cellular autophagy. Infect Immun. 2013;81:552–9. doi: 10.1128/IAI.00861-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y, Cho NH, Cho BA, Kim IS, Choi MS. Involvement of Ca2+ signaling in intracellular invasion of non-phagocytic host cells by Orientia tsutsugamushi. Microb Pathog. 2011;50:326–30. doi: 10.1016/j.micpath.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–70. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koraluru M, Bairy I, Varma M, Vidyasagar S. Diagnostic validation of selected serological tests for detecting scrub typhus. Microbiol Immunol. 2015;59:371–374. doi: 10.1111/1348-0421.12268. [DOI] [PubMed] [Google Scholar]
- Kramme S, An Le V, Khoa ND, Trin Le V, Tannich E, et al. Orientia tsutsugamushi bacteremia and cytokine levels in Vietnamese scrub typhus patients. J Clin Microbiol. 2009;47:586–9. doi: 10.1128/JCM.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kumar PS, Kaur G, Bhalla A, Sharma N, et al. Rare concurrent infection with scrub typhus, dengue and malaria in a young female. J Vector Borne Dis. 2014;51:71–2. [PubMed] [Google Scholar]
- Kuo CC, Huang JL, Ko CY, Lee PF, Wang HC. Spatial analysis of scrub typhus infection and its association with environmental and socioeconomic factors in Taiwan. Acta Trop. 2011;120:52–8. doi: 10.1016/j.actatropica.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Kuo CC, Huang JL, Shu PY, Lee PL, Kelt DA, et al. Cascading effect of economic globalization on human risks of scrub typhus and tick-borne rickettsial diseases. Ecol Appl. 2012;22:1803–16. doi: 10.1890/12-0031.1. [DOI] [PubMed] [Google Scholar]
- Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, et al. A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am J Trop Med Hyg. 2009;80:442–6. [PubMed] [Google Scholar]
- Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, et al. Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis. 2009;15:1127–9. doi: 10.3201/eid1507.080399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Chen YH, Lin JN, Chang LL, Chen WF, et al. Acute Q fever and scrub typhus, southern Taiwan. Emerg Infect Dis. 2009;15:1659–61. doi: 10.3201/eid1510.090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ji M, Hwang JH, Lee JY, Lee JH, et al. Acute cholecystitis in patients with scrub typhus. J Korean Med Sci. 2015;30:1698–700. doi: 10.3346/jkms.2015.30.11.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Shim SK, Song BG, Choi EN, Hwang KJ, et al. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector-Borne Zoonot Dis. 2011;11:209–214. doi: 10.1089/vbz.2009.0180. [DOI] [PubMed] [Google Scholar]
- Lee HW, Cho PY, Moon SU, Na BK, Kang YJ, et al. Current situation of scrub typhus in South Korea from 2001–2013. Parasit Vectors. 2015;8:238. doi: 10.1186/s13071-015-0858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Cho NH, Kim SY, Bang SY, Chu H, et al. Fibronectin facilitates the invasion of Orientia tsutsugamushi into host cells through interaction with a 56-kDa type-specific antigen. J Infect Dis. 2008;198:250–7. doi: 10.1086/589284. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee JH, Chung KM, Kim ES, Kwak YG, et al. Dynamics of clinical symptoms in patients with scrub typhus. Jpn J Infect Dis. 2013;66:155–7. doi: 10.7883/yoken.66.155. [DOI] [PubMed] [Google Scholar]
- Lee KY, Lee HS, Hong JH, Hur JK, Whang KT. Roxithromycin treatment of scrub typhus (tsutsugamushi disease) in children. Pediatr Infect Dis J. 2003;22:130–3. doi: 10.1097/01.inf.0000047864.80791.20. [DOI] [PubMed] [Google Scholar]
- Lee OH, Baek JH, Lee JS, Chung MH, Lee SM, et al. In vitro antagonism between cefotaxime and anti-rickettsial antibiotics against Orientia tsutsugamushi. Infect Chemother. 2014;46:189–93. doi: 10.3947/ic.2014.46.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim DM, Cho YS, Yoon SH, Shim SK. Usefulness of eschar PCR for diagnosis of scrub typhus. J Clin Microbiol. 2006;44:1169–71. doi: 10.1128/JCM.44.3.1169-1171.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kim MK, Kim MJ, Kang JS. Novel polysaccharide antigen of Orientia tsutsugamushi revealed by a monoclonal antibody. FEMS Microbiol Lett. 2009;297:95–100. doi: 10.1111/j.1574-6968.2009.01663.x. [DOI] [PubMed] [Google Scholar]
- Lee WS, Ou TY, Chen FL, Hsu CW, Jean SS. Co-infection with Orientia tsutsugamushi and Mycoplasma pneumoniae in a traveler. J Microbiol Immunol Infect. 2015;48:121–2. doi: 10.1016/j.jmii.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Lerdthusnee K, Khlaimanee N, Monkanna T, Sangjun N, Mungviriya S, et al. Efficiency of Leptotrombidium chiggers (Acari: Trombiculidae) at transmitting Orientia tsutsugamushi to laboratory mice. J Med Entomol. 2002;39:521–5. doi: 10.1603/0022-2585-39.3.521. [DOI] [PubMed] [Google Scholar]
- Lerdthusnee K, Nigro J, Monkanna T, Leepitakrat W, Leepitakrat S, et al. Surveys of rodent-borne disease in Thailand with a focus on scrub typhus assessment. Integr Zool. 2008;3:267–73. doi: 10.1111/j.1749-4877.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- Levine HD. Pathologic study of thirty-one cases of scrub typhus fever with especial reference to the cardiovascular system. Am Heart J. 1946;31:314–28. doi: 10.1016/0002-8703(46)90313-4. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Yousuf AA, Lerdthusnee K, Razee A, Chandranoi K, et al. Scrub typhus re-emergence in the Maldives. Emerg Infect Dis. 2003;9:1638–41. doi: 10.3201/eid0912.030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yang Z, Dong Z, Wang M. Meteorological factors and risk of scrub typhus in Guangzhou, southern China, 2006–2012. BMC Infect Dis. 2014;14:139. doi: 10.1186/1471-2334-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yang Z, Wang M. Scrub typhus rapidly increased in Guangzhou, Southern China, 2007–2012. Rev Inst Med Trop Sao Paulo. 2013;55:293–4. doi: 10.1590/S0036-46652013000400015. [DOI] [Google Scholar]
- Likeman R. Scrub typhus: a recent outbreak among military personnel in North Queensland. ADF Health. 2006;7:10–13. [Google Scholar]
- Lim C, Blacksell SD, Laongnualpanich A, Kantipong P, Day NP, et al. Optimal cutoff titers for indirect immunofluorescence assay for diagnosis of scrub typhus. J Clin Microbiol. 2015;53:3663–6. doi: 10.1128/JCM.01680-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Paris DH, Blacksell SD, Laongnualpanich A, Kantipong P, et al. How to determine the accuracy of an alternative diagnostic test when it is actually better than the reference tests: a re-evaluation of diagnostic tests for scrub typhus using Bayesian LCMs. PLoS One. 2015;10:e0114930. doi: 10.1371/journal.pone.0114930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PR, Tsai HP, Weng MH, Lin HC, Chen KC, et al. Field assessment of Orientia tsutsugamushi infection in small mammals and its association with the occurrence of human scrub typhus in Taiwan. Acta Trop. 2014;131:117–23. doi: 10.1016/j.actatropica.2013.11.029. [DOI] [PubMed] [Google Scholar]
- Luce-Fedrow A, Mullins K, Kostik AP, St John HK, Jiang J, et al. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Future Microbiol. 2015;10:537–64. doi: 10.2217/fmb.14.141. [DOI] [PubMed] [Google Scholar]
- Luksameetanasan R, Blacksell SD, Kalambaheti T, Wuthiekanun V, Chierakul W, et al. Patient and sample-related factors that effect the success of in vitro isolation of Orientia tsutsugamushi. Southeast Asian J Trop Med Public Health. 2007;38:91–6. [PubMed] [Google Scholar]
- Lyu Y, Tian L, Zhang L, Dou X, Wang X, et al. A case-control study of risk factors associated with scrub typhus infection in Beijing, China. PLoS One. 2013;8:e63668. doi: 10.1371/journal.pone.0063668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, et al. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J Assoc Physician I. 2006;54:619–21. [PubMed] [Google Scholar]
- Mahajan SK, Kaushik M, Raina R, Thakur P. Scrub typhus and malaria co-infection causing severe sepsis. Trop Doct. 2014;44:43–5. doi: 10.1177/0049475513512640. [DOI] [PubMed] [Google Scholar]
- Manosroi J, Chutipongvivate S, Auwanit W, Manosroi A. Early diagnosis of scrub typhus in Thailand from clinical specimens by nested polymerase chain reaction. Southeast Asian J Trop Med Public Health. 2003;34:831–8. [PubMed] [Google Scholar]
- Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359–64. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- McCulloch RN. Studies in the control of scrub typhus. Med J Aust. 1946;1:717–38. [PubMed] [Google Scholar]
- Min CK, Kwon YJ, Ha NY, Cho BA, Kim JM, et al. Multiple Orientia tsutsugamushi ankyrin repeat proteins interact with SCF1 ubiquitin ligase complex and eukaryotic elongation factor 1 alpha. PLoS One. 2014;9:e105652. doi: 10.1371/journal.pone.0105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura S, Ohta T, Tamura A. Comparison of in vitro susceptibilities of Rickettsia prowazekii, R. rickettsii, R. sibirica and R. tsutsugamushi to antimicrobial agents. Nippon Saikingaku Zasshi. 1989;44:717–721. doi: 10.3412/jsb.44.717. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Tamura A, Moriuchi H. In vitro reactivation of human immunodeficiency virus-1 upon stimulation with scrub typhus rickettsial infection. Am J Trop Med Hyg. 2003;68:557–561. doi: 10.4269/ajtmh.2003.68.557. [DOI] [PubMed] [Google Scholar]
- Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–9. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- Nagayo M, Miyagawa Y, Mitamura T, Imamura A. On the nymph and prosopon of the tsutsugamushi, Leptotrombidium akamushi, n. sp. (Trombidium Akamushi brumpt), carrier of the tsutsugamushi disease. J Exp Med. 1917;25:255–272. doi: 10.1084/jem.25.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayo M, Tamiya T, Mitamura T, Sato K. On the virus of tsutsugamushi disease and its demonstration by a new method. Jpn J Exp Med. 1930;8:309–318. [Google Scholar]
- Nakayama K, Kurokawa K, Fukuhara M, Urakami H, Yamamoto S, et al. Genome comparison and phylogenetic analysis of Orientia tsutsugamushi strains. DNA Res. 2010;17:281–91. doi: 10.1093/dnares/dsq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, et al. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res. 2008;15:185–99. doi: 10.1093/dnares/dsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal TJ, Barnett HC. The life cycle of the scrub typhus chigger mite, Trombicula akamushi. Ann Entomol Soc Am. 1961;54:196–203. doi: 10.1093/aesa/54.2.196. [DOI] [Google Scholar]
- Noad KB, Haymaker W. The neurological features of tsutsugamushi fever, with special reference to deafness. Brain. 1953;76:113–131. doi: 10.1093/brain/76.1.113. [DOI] [PubMed] [Google Scholar]
- Noh M, Lee Y, Chu C, Gwack J, Youn S-K, et al. Are there spatial and temporal correlations in the incidence distribution of scrub typhus in Korea? Osong Pub Health Res Perspect. 2013;4:39–44. doi: 10.1016/j.phrp.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, La Scola B, Audic S, Renesto P, Blanc G, et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2006;2:e76. doi: 10.1371/journal.pgen.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N. Aetiologie der Tsutsugamuchi-Krankheit: Rickettsia tsutsugamushi. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt I Orig. 1931;122:249–253. [Google Scholar]
- Ogawa M, Fukasawa M, Satoh M, Hanada K, Saijo M, et al. The intracellular pathogen Orientia tsutsugamushi responsible for scrub typhus induces lipid droplet formation in mouse fibroblasts. Microb Infect. 2014;16:962–6. doi: 10.1016/j.micinf.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Hagiwara T, Kishimoto T, Shiga S, Yoshida Y, et al. Scrub typhus in Japan: epidemiology and clinical features of cases reported in 1998. Am J Trop Med Hyg. 2002;67:162–5. doi: 10.4269/ajtmh.2002.67.162. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Ono T. Epidemiological characteristics of tsutsugamushi disease in Oita Prefecture, Japan: yearly and monthly occurrences of its infections and serotypes of its causative agent, Orientia tsutsugamushi, during 1984–2005. Microbiol Immunol. 2008;52:135–43. doi: 10.1111/j.1348-0421.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Fukuhara M, Shimada M, Tamura A. Phylogenetic position of Rickettsia tsutsugamushi and the relationship among its antigenic variants by analyses of 16S rRNA gene sequences. FEMS Microbiol Lett. 1995;125:299–304. doi: 10.1111/j.1574-6968.1995.tb07372.x. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Koyama Y, Urakami H, Fukuhara M, Tamura A, et al. Demonstration of antigenic and genotypic variation in Orientia tsutsugamushi which were isolated in Japan, and their classification into type and subtype. Microbiol Immunol. 1996;40:627–38. doi: 10.1111/j.1348-0421.1996.tb01120.x. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Nashimoto H, Ikeda H, Tamura A. Diversity of immunodominant 56-kDa type-specific antigen (TSA) of Rickettsia tsutsugamushi. Sequence and comparative analyses of the genes encoding TSA homologues from four antigenic variants. J Biol Chem. 1992;267:12728–35. [PubMed] [Google Scholar]
- Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT. Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg. 1980;29:989–97. [PubMed] [Google Scholar]
- Olson JG, Bourgeois AL, Fang RCY, Dennis DT (1981) Risk of relapse associated with doxycycline therapy for scrub typhus. In Burgdorfer W and Anacker RL (ed.), Rickettsiae and rickettsial diseases. New York, Academic Press, 201–210
- Ono Y, Ikegami Y, Tasaki K, Abe M, Tase C. Case of scrub typhus complicated by severe disseminated intravascular coagulation and death. Emerg Med Australas. 2012;24:577–80. doi: 10.1111/j.1742-6723.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- Osuga K, Kimura M, Goto H, Shimada K, Suto T. A case of tsutsugamushi disease probably contracted in Africa. Eur J Clin Microbiol Infect Dis. 1991;10:95–6. doi: 10.1007/BF01964418. [DOI] [PubMed] [Google Scholar]
- Otterdal K, Janardhanan J, Astrup E, Ueland T, Prakash JA, et al. Increased endothelial and macrophage markers are associated with disease severity and mortality in scrub typhus. J Infect. 2014;69:462–9. doi: 10.1016/j.jinf.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Paris DH, Aukkanit N, Jenjaroen K, Blacksell SD, Day NP. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect. 2009;15:488–95. doi: 10.1111/j.1469-0691.2008.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Blacksell SD, Nawtaisong P, Jenjaroen K, Teeraratkul A, et al. Diagnostic accuracy of a loop-mediated isothermal PCR assay for detection of Orientia tsutsugamushi during acute scrub typhus infection. PLoS Negl Trop Dis. 2011;5:e1307. doi: 10.1371/journal.pntd.0001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Blacksell SD, Newton PN, Day NP. Simple, rapid and sensitive detection of Orientia tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc Trop Med Hyg. 2008;102:1239–46. doi: 10.1016/j.trstmh.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–7. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lee JH, Jeong EJ, Kim JE, Hong SJ, et al. Rapid and simple identification of Orientia tsutsugamushi from other group rickettsiae by duplex PCR assay using groEL gene. Microbiol Immunol. 2005;49:545–9. doi: 10.1111/j.1348-0421.2005.tb03760.x. [DOI] [PubMed] [Google Scholar]
- Park JS, Jee YK, Lee KY, Kim KY, Myong NH, et al. Acute respiratory distress syndrome associated with scrub typhus: diffuse alveolar damage without pulmonary vasculitis. J Korean Med Sci. 2000;15:343–5. doi: 10.3346/jkms.2000.15.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Ha NY, Ryu B, Bang JH, Song H, et al. Urbanization of scrub typhus disease in South Korea. PLoS Negl Trop Dis. 2015;9:e0003814. doi: 10.1371/journal.pntd.0003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, et al. Trans-stadial and transovarial transmission of Orientia tsutsugamushi in Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae) J Med Entomol. 2009;46:1442–5. doi: 10.1603/033.046.0628. [DOI] [PubMed] [Google Scholar]
- Phasomkusolsil S, Tanskul P, Ratanatham S, Watcharapichat P, Phulsuksombati D, et al. Influence of Orientia tsutsugamushi infection on the developmental biology of Leptotrombidium imphalum and Leptotrombidium chiangraiensis (Acari: Trombiculidae) J Med Entomol. 2012;49:1270–1275. doi: 10.1603/ME12100. [DOI] [PubMed] [Google Scholar]
- Phetsouvanh R, Thojaikong T, Phoumin P, Sibounheuang B, Phommasone K, et al. Inter- and intra-operator variability in the reading of indirect immunofluorescence assays for the serological diagnosis of scrub typhus and murine typhus. Am J Trop Med Hyg. 2013;88:932–6. doi: 10.4269/ajtmh.12-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip CB. Tsutsugamushi disease in World War II. J Parasitol. 1948;34:169–91. doi: 10.2307/3273264. [DOI] [PubMed] [Google Scholar]
- Phommasone K, Paris DH, Anantatat T, Castonguay-Vanier J, Keomany S, et al. Concurrent infection with murine typhus and scrub typhus in southern Laos- the mixed and the unmixed. PLoS Negl Trop Dis. 2013;7:e2163. doi: 10.1371/journal.pntd.0002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongponratn E, Maneerat Y, Chaisri U, Wilairatana P, Punpoowong B, et al. Electron-microscopic examination of Rickettsia tsutsugamushi-infected human liver. Trop Med Int Health. 1998;3:242–8. doi: 10.1046/j.1365-3156.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Prakash JA, Abraham OC, Mathai E. Evaluation of tests for serological diagnosis of scrub typhus. Trop Doct. 2006;36:212–3. doi: 10.1258/004947506778604715. [DOI] [PubMed] [Google Scholar]
- Premaratna R., Chandrasena T. G. A. N., Dassayake A. S., Loftis A. D., Dasch G. A., de Silva H. J. Acute Hearing Loss Due to Scrub Typhus: A Forgotten Complication of a Reemerging Disease. Clinical Infectious Diseases. 2006;42(1):e6–8. doi: 10.1086/498747. [DOI] [PubMed] [Google Scholar]
- Rapmund G, Dohany AL, Manikumaran C, Chan TC. Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium (Leptotrombidium) arenicola Traub (Acarina: Trombiculidae) J Med Entomol. 1972;9:71–2. doi: 10.1093/jmedent/9.1.71. [DOI] [PubMed] [Google Scholar]
- Rapmund G, Upham RW, Jr, Kundin WD, Manikumaran C, Chan TC. Transovarial development of scrub typhus rickettsiae in a colony of vector mites. Trans R Soc Trop Med Hyg. 1969;63:251–8. doi: 10.1016/0035-9203(69)90155-2. [DOI] [PubMed] [Google Scholar]
- Rights FL, Smadel JE. Studies on scrub typhus; tsutsugamushi disease; heterogenicity of strains of R. tsutsugamushi as demonstrated by cross-vaccination studies. J Exp Med. 1948;87:339–51. doi: 10.1084/jem.87.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Ito S. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J Exp Med. 1979;150:703–8. doi: 10.1084/jem.150.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LW, Rapmund G, Gadigan FG. Sex ratios in Rickettsia tsutsugamushi-infected and noninfected colonies of Leptotrombidium (Acari: Trombiculidae) J Med Entomol. 1977;14:89–92. doi: 10.1093/jmedent/14.1.89. [DOI] [PubMed] [Google Scholar]
- Roberts LW, Robinson DM. Efficiency of transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium arenicola (Acari: Trombiculidae) J Med Entomol. 1977;13:493–496. doi: 10.1093/jmedent/13.4-5.493. [DOI] [PubMed] [Google Scholar]
- Rodkvamtook W, Prasartvit A, Jatisatienr C, Jatisatienr A, Gaywee J, et al. Efficacy of plant essential oils for the repellents against chiggers (Leptotrombidium imphalum) vector of scrub typhus. J Med Assoc Thai. 2012;95(Suppl 5):S103–6. [PubMed] [Google Scholar]
- Rodkvamtook W, Zhang Z, Chao CC, Huber E, Bodhidatta D, et al. Dot-ELISA rapid test using recombinant 56-kDa protein antigens for serodiagnosis of scrub typhus. Am J Trop Med Hyg. 2015;92:967–71. doi: 10.4269/ajtmh.14-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JY, Song BG, Park WI, Shin EH, Park C, et al. Coincidence between geographical distribution of Leptotrombidium scutellare and scrub typhus incidence in South Korea. PLoS One. 2014;9:e113193. doi: 10.1371/journal.pone.0113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. Drug-resistant scrub typhus: Paradigm and paradox. Parasitol Today. 1997;13:131–2. doi: 10.1016/S0169-4758(97)01020-X. [DOI] [PubMed] [Google Scholar]
- Santibáñez P, Palomar AM, Portillo A, Santibáñez S, Oteo JA. The role of chiggers as human pathogens. In: Samie A, editor. An Overview of Tropical Diseases. 2015. [Google Scholar]
- Sethi S, Prasad A, Biswal M, Hallur VK, Mewara A, et al. Outbreak of scrub typhus in North India: a re-emerging epidemic. Tropical Doctor. 2014;44:156–159. doi: 10.1177/0049475514523761. [DOI] [PubMed] [Google Scholar]
- Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58:208–10. [PubMed] [Google Scholar]
- Sharma PK, Ramakrishnan R, Hutin YJ, Barui AK, Manickam P, et al. Scrub typhus in Darjeeling, India: opportunities for simple, practical prevention measures. Trans R Soc Trop Med Hyg. 2009;103:1153–8. doi: 10.1016/j.trstmh.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Sheehy TW, Hazlett D, Turk RE. Scrub typhus: A comparison of chloramphenicol and tetracycline in its treatment. Arch Int Med. 1973;132:77–80. doi: 10.1001/archinte.1973.03650070069010. [DOI] [PubMed] [Google Scholar]
- Shin EH, Roh JY, Park WI, Song BG, Chang KS, et al. Transovarial transmission of Orientia tsutsugamushi in Leptotrombidium palpale (Acari: Trombiculidae) PLoS One. 2014;9:e88453. doi: 10.1371/journal.pone.0088453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A, Huxsoll DL, Dohany AL, Montrey RD, Werner RM, et al. Characterization of Rickettsia tsutsugamushi strains in two species of naturally infected, laboratory-reared chiggers. Am J Trop Med Hyg. 1982;31:395–402. doi: 10.4269/ajtmh.1982.31.395. [DOI] [PubMed] [Google Scholar]
- Shirai A, Robinson DM, Brown GW, Gan E, Huxsoll DL. Antigenic analysis by direct immunofluorescence of 114 isolates of Rickettsia tsutsugamushi recovered from febrile patients in rural Malaysia. Jp J Med Sci Biol. 1979;32:337–344. doi: 10.7883/yoken1952.32.337. [DOI] [PubMed] [Google Scholar]
- Shishido A. Strain variation of Rickettsia orientalis in the complement fixation test. Jp J Med Sci Biol. 1964;17:59–72. doi: 10.7883/yoken1952.17.59. [DOI] [PubMed] [Google Scholar]
- Silverman DJ, Wisseman CL. Comparative ultrastructural study on the cell envelopes of Rickettsia prowazekii, Rickettsia rickettsii and Rickettsia tsutsugamushi. Infect Immun. 1978;21:1020–1023. doi: 10.1128/iai.21.3.1020-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Eldin C, Kowalczewska M, Raoult D. Axenic culture of fastidious and intracellular bacteria. Trends Microbiol. 2013;21:92–9. doi: 10.1016/j.tim.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Singhsilarak T, Leowattana W, Looareesuwan S, Wongchotigul V, Jiang J, et al. Short report: detection of Orientia tsutsugamushi in clinical samples by quantitative real-time polymerase chain reaction. Am J Trop Med Hyg. 2005;72:640–1. [PubMed] [Google Scholar]
- Sirisanthana V, Puthanakit T, Sirisanthana T. Epidemiologic, clinical and laboratory features of scrub typhus in thirty Thai children. Pediatr Infect Dis J. 2003;22:341–5. doi: 10.1097/01.inf.0000059400.23448.57. [DOI] [PubMed] [Google Scholar]
- Smadel JE, Bailey CA, Diercks FH. Chloramphenicol (chloromycetin) in the chemoprophylaxis of scrub typhus (tsutsugamushi disease) IV. Relapses of scrub typhus in treated volunteers and their prevention. Am J Hyg. 1950;51:229–241. [Google Scholar]
- Smadel JE, Ley HL, Jr, Diercks RH, Cameron JA. Persistence of Rickettsia tsutsugamushi in tissues of patients recovered from scrub typhus. Am J Hyg. 1952;56:294–302. doi: 10.1093/oxfordjournals.aje.a119553. [DOI] [PubMed] [Google Scholar]
- Smadel JE, Woodward TE, Ley HL, Jr, Lewthwaite R. Chloramphenicol (chloromycetin) in the treatment of tsutsugamushi disease (scrub typhus) J Clin Invest. 1949;28:1196–1215. doi: 10.1172/JCI102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smadel JE, Woodward TE, Ley HL, Jr, Philip CB, Traub R, et al. Chloromycetin in the treatment of scrub typhus. Science. 1948;108:160–1. doi: 10.1126/science.108.2798.160. [DOI] [PubMed] [Google Scholar]
- Son D, Chung MH. In vitro synergism between chloroquine and antibiotics against Orientia tsutsugamushi. Infect Chemother. 2014;46:182–8. doi: 10.3947/ic.2014.46.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Lee C, Chang WH, Choi SW, Choi JE, et al. Short-course doxycycline treatment versus conventional tetracycline therapy for scrub typhus: a multicenter randomized trial. Clin Infect Dis. 1995;21:506–510. doi: 10.1093/clinids/21.3.506. [DOI] [PubMed] [Google Scholar]
- Song SW, Kim KT, Ku YM, Park SH, Kim YS, et al. Clinical role of interstitial pneumonia in patients with scrub typhus: a possible marker of disease severity. J Korean Med Sci. 2004;19:668–73. doi: 10.3346/jkms.2004.19.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonthayanon P, Chierakul W, Wuthiekanun V, Blacksell SD, Pimda K, et al. Rapid diagnosis of scrub typhus in rural Thailand using polymerase chain reaction. Am J Trop Med Hyg. 2006;75:1099–102. [PubMed] [Google Scholar]
- Sonthayanon P, Chierakul W, Wuthiekanun V, Limmathurotsakul D, Amornchai P, et al. Molecular confirmation of co-infection by pathogenic Leptospira spp. and Orientia tsutsugamushi in patients with acute febrile illness in Thailand. Am J Trop Med Hyg. 2013;89:797–9. doi: 10.4269/ajtmh.13-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonthayanon P, Chierakul W, Wuthiekanun V, Phimda K, Pukrittayakamee S, et al. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J Clin Microbiol. 2009;47:430–4. doi: 10.1128/JCM.01927-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonthayanon P, Peacock SJ, Chierakul W, Wuthiekanun V, Blacksell SD, et al. High rates of homologous recombination in the mite endosymbiont and opportunistic human pathogen Orientia tsutsugamushi. PLoS Negl Trop Dis. 2010;4:e752. doi: 10.1371/journal.pntd.0000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Marana DP, Carter JM, Roe BA, Mardis E, et al. (1990) The 56-kilodalton major protein antigen of Rickettsia tsutsugamushi: molecular cloning and sequence analysis of the sta56 gene and precise identification of a strain-specific epitope. Infect Immun 58: 2076–2084. [DOI] [PMC free article] [PubMed]
- Strickman D, Tanskul P, Eamsila C, Kelly DJ. Prevalence of antibodies to rickettsiae in the human population of suburban Bangkok. Am J Trop Med Hyg. 1994;51:149–53. doi: 10.4269/ajtmh.1994.51.149. [DOI] [PubMed] [Google Scholar]
- Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–70. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Murata M, Misumi H, Hori E, Kawamura A, Jr, et al. Failed vertical transmission of Rickettsia tsutsugamushi (Rickettsiales: Rickettsiaceae) acquired from rickettsemic mice by Leptotrombidium pallidum (Acari: Trombiculidae) J Med Entomol. 1994;31:212–6. doi: 10.1093/jmedent/31.2.212. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Murata M, Nogami S, Hori E, Kawamura A, Jr, et al. Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium pallidum successively reared in the laboratory. Jpn J Exp Med. 1988;58:213–8. [PubMed] [Google Scholar]
- Tamura A, Ohashi N, Urakami H, Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45:589–91. doi: 10.1099/00207713-45-3-589. [DOI] [PubMed] [Google Scholar]
- Tamura A, Ohashi N, Urakami H, Takahashi K, Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985;48:671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Urakami H, Ohashi N. A comparative view of Rickettsia tsutsugamushi and the other groups of rickettsiae. Eur J Epidemiol. 1991;7:259–269. doi: 10.1007/BF00145675. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanskul P, Linthicum KJ, Watcharapichat P, Phulsuksombati D, Mungviriya S, et al. A new ecology for scrub typhus associated with a focus of antibiotic resistance in rice farmers in Thailand. J Med Entomol. 1998;35:551–5. doi: 10.1093/jmedent/35.4.551. [DOI] [PubMed] [Google Scholar]
- Tantibhedhyangkul W, Angelakis E, Tongyoo N, Newton PN, Moore CE, et al. Intrinsic fluoroquinolone resistance in Orientia tsutsugamushi. Int J Antimicrob Agents. 2010;35:338–41. doi: 10.1016/j.ijantimicag.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, et al. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis. 2011;5:e1028. doi: 10.1371/journal.pntd.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi) PLoS Negl Trop Dis. 2015;9:e0003971. doi: 10.1371/journal.pntd.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiga JW, Mutai BK, Eyako WK, Ng'ang'a Z, Jiang J, et al. High seroprevalence of antibodies against spotted fever and scrub typhus bacteria in patients with febrile illness, Kenya. Emerg Infect Dis. 2015;21:688–91. doi: 10.3201/eid2104.141387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R, Wisseman CL., Jr Ecological considerations in scrub typhus. 2. Vector species. Bull World Health Org. 1968;39:219–30. [PMC free article] [PubMed] [Google Scholar]
- Traub R, Wisseman CL., Jr The ecology of chigger-borne rickettsiosis (scrub typhus) J Med Entomol. 1974;11:237–303. doi: 10.1093/jmedent/11.3.237. [DOI] [PubMed] [Google Scholar]
- Traub R, Wisseman CL, Jr, Jones MR, O'Keefe JJ. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann N Y Acad Sci. 1975;266:91–114. doi: 10.1111/j.1749-6632.1975.tb35091.x. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Lay CJ, Wang CL, Ho YH, Wang LS, et al. Levofloxacin versus tetracycline antibiotics for the treatment of scrub typhus. Int J Infect Dis. 2010;14:e62–7. doi: 10.1016/j.ijid.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Tsai PJ, Yeh HC. Scrub typhus islands in the Taiwan area and the association between scrub typhus disease and forest land use and farmer population density: geographically weighted regression. BMC Infect Dis. 2013;13:191. doi: 10.1186/1471-2334-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay RW, Chang FY. Serious complications in scrub typhus. J Microbiol Immunol Infect. 1998;31:240–4. [PubMed] [Google Scholar]
- Twartz JC, Shirai A, Selvaraju G, Saunders JP, Huxsoll DL, et al. Doxycycline propylaxis for human scrub typhus. J Infect Dis. 1982;146:811–8. doi: 10.1093/infdis/146.6.811. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services 2009. Rickettsial agents. In: Wilson, DE, Casey Chosewood, L (eds.) Biosafety in Microbiological and Biomedical Laboratories. 5th ed.
- Upham RW, Hubert AA, Phang OW, Mat YB, Rapmund G. Distribution of Leptotrombidium (Leptotrombidium) Arenicola (Acarina: Trombiculidae) on the ground in West Malaysia. J Med Entomol. 1971;8:401–406. doi: 10.1093/jmedent/8.4.401. [DOI] [PubMed] [Google Scholar]
- Urakami H, Takahashi M, Hori E, Tamura A. An ultrastructural study of vertical transmission of Rickettsia tsutsugamushi during oogenesis and spermatogenesis in Leptotrombidium pallidum. Am J Trop Med Hyg. 1994;50:219–28. doi: 10.4269/ajtmh.1994.50.219. [DOI] [PubMed] [Google Scholar]
- Urakami H, Takahashi M, Murata M, Tamura A. Electron microscopic study of the distribution and the vertical transmission of Rickettsia tsutsugamushi in Leptotrombidium pallidum. Jp J Med Sci Biol. 1994;47:127–39. doi: 10.7883/yoken1952.47.127. [DOI] [PubMed] [Google Scholar]
- Urakami H, Tsuruhara T, Tamura A. Intranuclear Rickettsia tsutsugamushi in cultured mouse fibroblasts (L cells) Microbiol Immunol. 1982;26:445–7. doi: 10.1111/j.1348-0421.1982.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Urakami H, Tsuruhara T, Tamura A. Electron microscopic studies on intracellular multiplication of Rickettsia tsutsugamushi in L cells. Microbiol Immunol. 1984;28:1191–201. doi: 10.1111/j.1348-0421.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Vallée J, Thaojaikong T, Moore CE, Phetsouvanh R, Richards AL, et al. Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane City, Lao PDR. PLoS Negl Trop Dis. 2010;4:e909. doi: 10.1371/journal.pntd.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Peenen PF, Lien JC, Santana FJ, See R. Correlation of chigger abundance with temperature at a hyperendemic focus of scrub typhus. J Parasitol. 1976;62:653–4. doi: 10.2307/3279442. [DOI] [PubMed] [Google Scholar]
- Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, et al. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J Infect. 2006;52:56–60. doi: 10.1016/j.jinf.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Viebrock L, Evans SM, Beyer AR, Larson CL, Beare PA, et al. Orientia tsutsugamushi ankyrin repeat-containing protein family members are Type 1 secretion system substrates that traffic to the host cell endoplasmic reticulum. Front Cell Infect Microbiol. 2014;4:186. doi: 10.3389/fcimb.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JS, Chan CT, Manikumaran C, Elisberg BL. Attempts to infect and demonstrate transovarial transmission of R. tsutsugamushi in three species of leptotrombidium mites. Ann N Y Acad Sci. 1975;266:80–90. doi: 10.1111/j.1749-6632.1975.tb35090.x. [DOI] [PubMed] [Google Scholar]
- Walker JS, Gan E, Chan Teik C, Muul I. Involvement of small mammals in the transmission of scrub typhus in Malaysia: isolation and serological evidence. Trans R Soc Trop Med Hyg. 1973;67:838–45. doi: 10.1016/0035-9203(73)90012-6. [DOI] [PubMed] [Google Scholar]
- Wang CC, Liu SF, Liu JW, Chung YH, Su MC, et al. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76:1148–52. [PubMed] [Google Scholar]
- Wang CL, Yang KD, Cheng SN, Chu ML. Neonatal scrub typhus: a case report. Pediatrics. 1992;89:965–8. [PubMed] [Google Scholar]
- Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. 1996;348:86–89. doi: 10.1016/S0140-6736(96)02501-9. [DOI] [PubMed] [Google Scholar]
- Watt G, Jongsakul K, Chouriyagune C, Paris R. Differentiating dengue virus infection from scrub typhus in Thai adults with fever. Am J Trop Med Hyg. 2003;68:536–8. doi: 10.4269/ajtmh.2003.68.536. [DOI] [PubMed] [Google Scholar]
- Watt G, Jongsakul K, Suttinont C. Possible scrub typhus coinfections in Thai agricultural workers hospitalized with leptospirosis. Am J Trop Med Hyg. 2003;68:89–91. [PubMed] [Google Scholar]
- Watt G, Kantipong P, Burnouf T, Shikuma C, Philpott S. Natural scrub typhus antibody suppresses HIV CXCR4(X4) viruses. Infect Dis Rep. 2013;5:e8. doi: 10.4081/idr.2013.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G, Kantipong P, De Souza M, Chanbancherd P, Jongsakul K, et al. HIV-1 suppression during acute scrub-typhus infection. Lancet. 2000;356:475–9. doi: 10.1016/S0140-6736(00)02557-5. [DOI] [PubMed] [Google Scholar]
- Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, et al. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. 2000;356:1057–61. doi: 10.1016/S0140-6736(00)02728-8. [DOI] [PubMed] [Google Scholar]
- Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–36. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- Watthanaworawit W, Turner P, Turner C, Tanganuchitcharnchai A, Jintaworn S, et al. Diagnostic accuracy assessment of immunochromatographic tests for the rapid detection of antibodies against Orientia tsutsugamushi using paired acute and convalescent specimens. Am J Trop Med Hyg. 2015;93:1168–1171. doi: 10.4269/ajtmh.15-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watthanaworawit W, Turner P, Turner C, Tanganuchitcharnchai A, Richards AL, et al. A prospective evaluation of real-time PCR assays for the detection of Orientia tsutsugamushi and Rickettsia spp. for early diagnosis of rickettsial infections during the acute phase of undifferentiated febrile illness. Am J Trop Med Hyg. 2013;89:308–310. doi: 10.4269/ajtmh.12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Huang Y, Luo L, Xiao X, Liu L, et al. Rapid increase of scrub typhus: an epidemiology and spatial-temporal cluster analysis in Guangzhou City, Southern China, 2006–2012. PLoS One. 2014;9:e101976. doi: 10.1371/journal.pone.0101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Luo L, Jing Q, Li X, Huang Y, et al. A city park as a potential epidemic site of scrub typhus: a case-control study of an outbreak in Guangzhou, China. Parasit Vectors. 2014;7:513. doi: 10.1186/s13071-014-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YF, Chiu CT, Lai YF, Lai CH, Lin HH. Successful treatment of septic shock and respiratory failure due to leptospirosis and scrub typhus coinfection with penicillin, levofloxacin, and activated protein C. J Microbiol Immunol Infect. 2012;45:251–4. doi: 10.1016/j.jmii.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Weitzel Thomas, Dittrich Sabine, López Javier, Phuklia Weerawat, Martinez-Valdebenito Constanza, Velásquez Katia, Blacksell Stuart D., Paris Daniel H., Abarca Katia. Endemic Scrub Typhus in South America. New England Journal of Medicine. 2016;375(10):954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- Wongprompitak P, Duong V, Anukool W, Sreyrath L, Mai TT, et al. Orientia tsutsugamushi, agent of scrub typhus, displays a single metapopulation with maintenance of ancestral haplotypes throughout continental South East Asia. Infect Genet Evol. 2015;31:1–8. doi: 10.1016/j.meegid.2015.01.005. [DOI] [PubMed] [Google Scholar]
- World Health Organization Department of Communicable Disease Surveillance and Response . WHO Recommended Surveillance Standards Vol. 2. Geneva: World Health Organization; 1999. [Google Scholar]
- Wright JD, Hastriter MW, Robinson DM. Observations on the ultrastructure and distribution of Rickettsia Tsutsugamushi in naturally infected Leptotrombidium (Leptotrombidium) arenicola (Acari: Trombiculidae) J Med Entomol. 1984;21:17–27. doi: 10.1093/jmedent/21.1.17. [DOI] [PubMed] [Google Scholar]
- Wu YC, Qian Q, Magalhaes RJS, Han Z-H, Haque U, et al. Rapid increase in scrub typhus incidence in mainland China, 2006–2014. Am J Trop Med Hyg. 2016;94:532–6. doi: 10.4269/ajtmh.15-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Minamishima Y. Serodiagnosis of tsutsugamushi fever (scrub typhus) by the indirect immunoperoxidase technique. J Clin Microbiol. 1982;15:1128–1132. doi: 10.1128/jcm.15.6.1128-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Liu J, Wang XJ, Ma W, Jia CX, et al. Effects of meteorological factors on scrub typhus in a temperate region of China. Epidemiol Infect. 2014;142:2217–26. doi: 10.1017/S0950268813003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TH, Chang CT, Lin JL, Jiang JR, Lee KF. Scrub typhus: a frequently overlooked cause of acute renal failure. Ren Fail. 2003;25:397–410. doi: 10.1081/JDI-120021152. [DOI] [PubMed] [Google Scholar]
- Yun JH, Koh YS, Lee KH, Hyun JW, Choi YJ, et al. Chemokine and cytokine production in susceptible C3H/HeN mice and resistant BALB/c mice during Orientia tsutsugamushi infection. Microbiol Immunol. 2005;49:551–7. doi: 10.1111/j.1348-0421.2005.tb03761.x. [DOI] [PubMed] [Google Scholar]
- Zhang Meng, Wang Xian-Jun, Ding Lei, Zhao Zhong-Tang, Li Zhong, Ding Shu-Jun. Scrub Typhus: Surveillance, Clinical Profile and Diagnostic Issues in Shandong, China. The American Journal of Tropical Medicine and Hygiene. 2012;87(6):1099–1104. doi: 10.4269/ajtmh.2012.12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Song H, Liu Y, Li Q, Wang Y, et al. Scrub typhus in previously unrecognized areas of endemicity in China. J Clin Microbiol. 2010;48:1241–44. doi: 10.1128/JCM.01784-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WY, Wang LY, Ding F, Hu WB, Soares Magalhaes RJ, et al. Scrub typhus in mainland China, 2006–2012: the need for targeted public health interventions. PLoS Negl Trop Dis. 2013;7:e2493. doi: 10.1371/journal.pntd.0002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Yang HL, Bi ZW, Kou ZQ, Zhang LY, et al. Epidemic characteristics and spatio-temporal patterns of scrub typhus during 2006–2013 in Tai'an, Northern China. Epidemiol Infect. 2015;143:2451–8. doi: 10.1017/S0950268814003598. [DOI] [PMC free article] [PubMed] [Google Scholar]


