The collection and evaluation of synovial fluid in veterinary medicine was first used for the diagnosis of large animal lameness and joint disease. With the growth of knowledge and sophistication in the medical, radiographic, and orthopedic specialties, our focus in small animal arthrology and orthopedics expanded from fracture and cruciate repair to include the evaluation of degenerative, immune-mediated, infectious, and noninflammatory joint diseases. In the laboratory, methods for small animal specimens were modified to accommodate the smaller sample volumes. Concurrently, there has been a rapid increase in the number of serologic tests for the detection of infectious agents and immune disorders. From these beginnings, veterinarians can now recognize and treat an expanding list of joint diseases.
Indications
Arthrocentesis and joint fluid analysis are integral to the clinical evaluation of not only primary joint disorders but systemic diseases in which joint effusion is part of the clinical picture. The technique of arthrocentesis to acquire synovial fluid for analysis is no more difficult or imbued with risk than pleurocentesis or abdominocentesis. Synovial fluid analysis is also recommended in disorders characterized by persistent or fluctuating fever of unknown origin, shifting leg lameness, or generalized malaise in which arthralgia is suspected. In general, joints that contain excessive fluid should be sampled; however, if none exist, at least two to three joints should be sampled, especially the carpal and tarsal joints [1]. All cases of lingering lameness and those unresponsive to nonsteroidal anti-inflammatory drugs should have synovial fluid analyzed to rule out a chronic infectious process [2]. Arthrocentesis and examination of the synovial fluid can confirm the presence of arthritis, characterize the response as inflammatory or noninflammatory, and, in some circumstances, reveal the etiologic agent.
Anatomy and physiology
To understand the formation of joint effusions, knowledge of the anatomy and physiology of healthy joints is important [3]. Joints are made up of two opposing cartilage surfaces surrounded and supported by a multilayer capsule and bathed in synovial fluid. The proteoglycan matrix and collagen of articular cartilage are synthesized and maintained by chondrocytes located in lacunar spaces embedded within the cartilage. Proteoglycan consists of a polypeptide core with one or more negatively charged glycosaminoglycan (GAG) side chains. The GAGs of articular cartilage include keratin sulfate (KS), chondroitin sulfate (CS), and heparan sulfate. The major proteoglycan of articular cartilage is aggrecan, a large polymer consisting of numerous proteoglycan moieties noncovalently bound to a nonsulfated GAG, hyaluronan. Type II collagen predominates in articular cartilage, with smaller amounts of types VI, IX, X, and V/XI. The rigid collagen framework restricts the expansion of hydrophilic proteoglycans, providing articular cartilage with its unique ability to withstand compressive forces. As a result of normal cartilage maintenance, degradative enzymes, such as stromelysin (MMP-3), and proteoglycan fragments can be detected in synovial fluid. Assays for these enzymes are not readily available for diagnostic purposes.
The joint capsule is made up of three layers. The outermost fibrous layer provides stability and flexibility to the joint. Next, the subsynovium contains a vascular and neural network enmeshed in a loose fibrous connective tissue. These vessels are the source of the plasma ultrafiltrate that becomes synovial fluid (Fig. 1 ). The inner synovial lining consists of two cell types: type A synoviocytes, macrophage-like cells with phagocytic function, and type B synoviocytes, fibroblast-like cells that produce hyaluronic acid. Together, these cells prevent large molecules, such as plasma proteins, from entering the joint fluid and participate in the maintenance of articular cartilage.
Fig. 1.
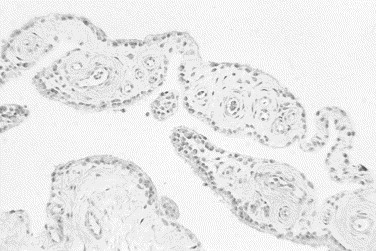
Histologic section of normal synovial tissue. Villous projections of connective tissue are covered by synovial membrane consisting of low cuboidal epithelial cells. Numerous blood vessels are noted within the connective tissue layer (hematoxylin–eosin stain, original magnification ×10).
Synovial fluid is an ultrafiltrate of plasma with the addition of hyaluronic acid, which provides the fluid with its viscous nature. Synovial fluid supplies nutrients to articular cartilage and functions as a boundary lubricant for periarticular tissues. During non-load-bearing motion, a synovial glycoprotein, lubricin, reduces friction between the surfaces by adhering to articular cartilage. During high load-bearing motion, the major joint lubricant is provided by fluid squeezed out of the cartilage matrix.
Pathogenesis of joint effusions
The formation of abnormal synovial effusions is similar whether it is mediated by cartilage injury that occurs in degenerative joint disease or by the synovitis of immune-mediated arthritis and differs mostly in the character and severity of the cellular infiltrate and articular damage. In response to an insult, chondrocytes and synoviocytes release cytokines, which causes vasodilation of the subsynovial capillaries, leading to increased vascular permeability and extravasation of fluid, protein, and inflammatory cells into the joint space. The newly arrived leukocytes promote further inflammatory changes and cause the release of degradative enzymes from multiple cell types. The type and number of leukocytes that infiltrate the synovium and migrate into synovial fluid define the cellular components of the joint effusion and the clinical features of the arthritis [3], [4]. The following list summarizes the causes of inflammatory and noninflammatory joint diseases in dogs and cats [5]:
Laboratory evaluation of synovial fluid
The laboratory assessment of synovial fluid includes an evaluation of physical characteristics, chemical tests, cell counts, and differential count [30]. In some cases, microbial cultures for infectious agents or serologic tests for infectious or immunologic diseases are indicated. The history, presentation, radiographic findings, and amount of fluid collected determine which of these tests are to be done.
The collection process can affect the synovial fluid results and interpretation. Hemodilution of the specimen by inadvertent penetration of a blood vessel can alter the total nucleated cell counts (TNCCs) and differential cell count. An important first step in arthrocentesis is the identification of anatomic landmarks surrounding the involved joint [30]. If an adequate sample volume is obtained, an aliquot should be put in a pediatric EDTA tube, because fluid from abnormal joints is more likely to clot. Fluid samples for cell counts and cytologic assessment can be stored for 24 hours in the refrigerator, but it is best to make slides immediately [9]. If only a few drops of fluid are collected, most would agree that preparation of stained slides for microscopic evaluation provides the most useful information to the diagnostic process [19], [30], [35].
Physical features
Evaluation of physical features includes a visual examination of color, turbidity, viscosity, and volume. Normal synovial fluid in the dog and cat is clear, colorless, viscous, free of flocculent debris ,and does not clot. Volume is usually noted subjectively rather than quantitatively, because joint aspiration in most dogs or cats yields less than 0.1 to 0.25 mL [30], [32]. Joint disease can be accompanied by an increase or decrease in synovial fluid volume. Increased volume is usually noted during the physical examination as joint distention. A dry or nearly dry joint tap usually indicates reduced volume.
The gross appearance of joint fluid and the interpretation of changes in appearance are similar to those that are used for other cavity fluids (Fig. 2 ) [6]. Normal joint fluid is clear and colorless. Red or red-tinged fluid indicates hemorrhage in the joint associated with trauma or inflammation or hemorrhage that has occurred during the collection process. Xanthochromia is a yellow-orange discoloration that indicates prior hemorrhage and hemoglobin breakdown. White or light yellow coloration or sediment indicates an increase in the nucleated cell count because of inflammation, sepsis, or neoplasia, or it may indicate crystal formation. An increase in turbidity is caused by suspended particulates, such as red blood cells, white blood cells, organisms, fibrin, neoplastic cells, or crystals. Alterations in color or turbidity caused by crystals or neoplastic cells are extremely rare.
Fig. 2.
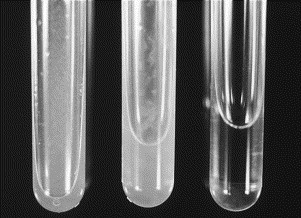
Samples of normal and abnormal synovial fluid. Normal synovial fluid is clear and colorless (right). Center and left tubes contain abnormal synovial fluid that is turbid and contains flocculent debris. Turbidity is caused by the presence of cells, fibrin, bacteria, or, on rare occasions, crystals.
Synovial fluid from normal joints does not clot in a tube or syringe but does tend to form a gel. It is important that gel formation not be confused with a clot. Agitation of the gel causes the sample to return to its fluid state. This reversible fluid-gel formation is called thixotropism [33].
Normal viscosity of synovial fluid is a result of the amount and polymerization of hyaluronic acid, which is a glycoprotein [9]. Viscosity is an indicator of the lubricating properties of the fluid in the joint, and decreased viscosity can be caused by several factors. Hyaluronic acid content can be reduced by decreased production as a result of synovial membrane damage, dilution by the influx of plasma or fluid, or degradation by white blood cells or bacteria. Intra-articular injection of drugs or joint lavage can also reduce fluid viscosity. Viscosity is usually assessed by visual observation (Fig. 3 ) [6]. A drop of fluid between the thumb and finger should form a strand that is at least 1 inch long when the digits are pulled apart. A drop from a needle and syringe should produce a strand at least 1 inch long before it breaks. Hyaluronic acid content can be assessed semiquantitatively by the mucin clot test.
Fig. 3.

Synovial fluid viscosity can be assessed during collection. A drop of fluid between the thumb and forefinger or dripped from a needle or pipette should form a strand at least 1 inch long. A sample of normal synovial fluid forms a strand 2 inches long between the pipette tip and the forefinger.
Nucleated cell counts
If there is adequate sample volume, enumeration of cells per microliter of the fluid is useful and can be a significant discriminator in the diagnostic process. Total nucleated cell counts (TNCCs) are extremely valuable in separating inflammatory and noninflammatory arthropathies and in differentiating among the various causes of inflammatory arthritis. Most laboratories use a reference value of less than 3000 cells per milliliter for the TNCC [6], [9]. Sequential TNCC counts in joint fluid are also useful in following the response to treatment in a particular joint. Red blood cell (RBC) counts provide little useful information.
TNCC counts can be determined by manual methods using a hemacytometer or by electronic cell counters. The viscosity and hyaluronic acid content of synovial fluid can adversely affect both methods [34]. The usual diluting fluid used for white blood cell (WBC) counts in blood cannot be used for synovial fluid because it causes acid precipitation of hyaluronic acid. The viscous nature of many synovial fluid samples causes inaccuracies in the pipetting and diluting of samples in either method. WBC counts generated by electronic counters are usually higher than those counted by manual methods, but the difference is not enough to alter interpretation [34], [46]. Several studies have indicated that the addition of hyaluronidase to the fluid before counting improves the consistency of TNCC counts and improves the quality of smears [9], [34]. This enzyme markedly reduces the viscosity, decreases the entrapment of cells, and produces a more random distribution of cells in the fluid. One or two drops of a 150-U/mL solution of hyaluronidase are added to a small aliquot of the synovial fluid. Within a few minutes, the fluid viscosity diminishes and the cells can be counted. It is important to realize that reducing the viscosity allows for rapid sedimentation of the nucleated cells. Samples treated with the enzyme must be mixed to resuspend cells before TNCC enumeration or preparation of smears. The addition of hyaluronidase also interferes with the mucin clot test.
Cell identification and differential cell counts
Smears of synovial fluid can be made using the same techniques employed for blood or cavity fluids. Fluid viscosity can present some problems that affect slide quality. The hyaluronic acid content of normal or noninflammatory effusions causes the background staining to appear pink and fine to coarsely granular and to contain numerous crescents or folds (Fig. 4 ) [6]. The background material causes many of the nucleated cells to be small, darkly stained, and difficult to recognize [33]. Neutrophils can become so rounded and dark that they cannot be distinguished from lymphocytes. Edges of the smear are the best places to search for identifiable cells. In samples with increased cell counts and normal viscosity, the nucleated cells form rows (Fig. 5 ). If the fluid is viscous, the spreader slide should be held at a low angle to spread the fluid over a larger area [9].
Fig. 4.
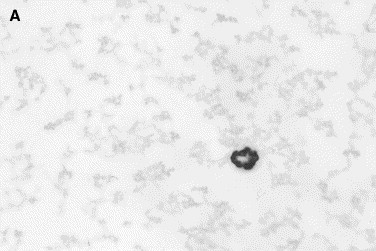

Normal canine synovial fluid. (A) The hyaluronic acid content in normal synovia produces a coarse granular pink background that highlights the cells. (B) The crescents are artifactual folds in the background material that indicate a normal content of hyaluronic acid in the sample (Wright stain, original magnification ×1000).
Fig. 5.
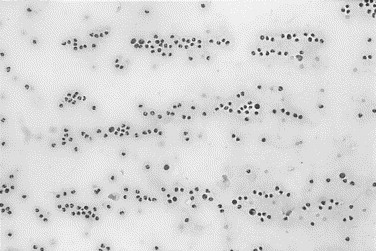
Canine synovial fluid with inflammatory arthritis. Leukocytes are increased and are arranged in rows. This leukocyte distribution indicates that the effusion contains adequate amounts of hyaluronic acid.
In synovial fluids with reduced glycoprotein or hyaluronic acid content, the dense pink granular background staining is reduced or may be nonexistent [6]. Therefore, the microscopic assessment always includes an evaluation of the granularity and staining intensity of the background, because it is an indicator of the glycoprotein or hyaluronic acid content.
RBCs are rare in normal joint fluid. If present in increased numbers, joint trauma, hemorrhage associated with inflammation, or hemorrhage as a result of arthrocentesis should be considered. In the latter instance, platelets are usually noted among the RBCs. With long-standing or resolving joint hemorrhage, macrophages phagocytose RBCs and accumulate hemoglobin breakdown pigment, which appears as blue-black coarse intracytoplasmic granules.
Nucleated cells recognized frequently in synovial fluid include neutrophils, lymphocytes, monocytes, and macrophages [9]. These cells are seen in fluids from normal as well as diseased joints. With some experience, the density of the nucleated cells on stained slides can be used to categorize the TNCC as normal, increased, or markedly increased. In a recent study, smears of normal synovial fluid revealed one to three nucleated cells per high-power field (HPF) (100× oil-immersion objective). Each nucleated cell per HPF was equivalent to 1000 cells per milliliter [35]. Less than 10% of the nucleated cells in normal synovial fluid are neutrophils [6], [19]. These neutrophils are identical to those seen in blood and other body fluids. Neutrophils have a segmented nucleus in a pale pink or light blue cytoplasm (Fig. 6 ). Neutrophil morphology is examined for evidence of degenerative changes such as vacuolation and basophilia, which are frequently associated with the presence of infectious agents (Fig. 7 ). Neutrophil cytoplasms should be examined for the presence of microorganisms. The remaining cells (90%) in normal joint fluid consist of small lymphocytes, monocytes, and macrophages (see Fig. 6). A few synovial membrane cells can be seen. Lymphocytes are smaller than neutrophils and have a thin rim of light blue cytoplasm and a smooth, dark purple, round nucleus (see Fig. 6). Monocytes have an abundant blue-gray cytoplasm surrounding a nucleus that can vary in shape and appear as indented, multilobulated, or band shaped (Fig. 8 ). Macrophages are larger than neutrophils and have a blue-gray vacuolated cytoplasm and an oval or indented nucleus. Phagocytosed cell debris is noted occasionally in the cytoplasm (Fig. 9 ) and is especially prominent in degenerative joint disease and resolving or chronic inflammation. Infectious agents, especially fungi and protozoa, are sometimes found within macrophage cytoplasms.
Fig. 6.
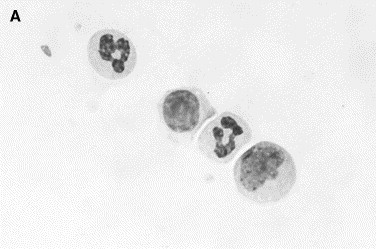
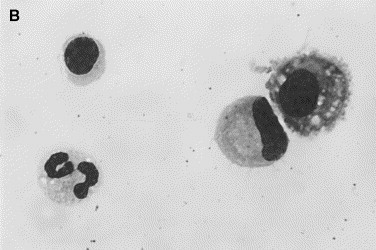
Normal synovial fluid. Nucleated cells in normal synovia are predominantly mononuclear cells consisting of small lymphocytes, monocytes, and macrophages. (A) Two neutrophils, a lymphocyte (center), and a monocyte (lower right). (B) A lymphocyte and neutrophil (left) are compared with a monocyte and macrophage (right). The latter are larger and have a vacuolated granular cytoplasm (Wright stain, original magnification ×1000).
Fig. 7.
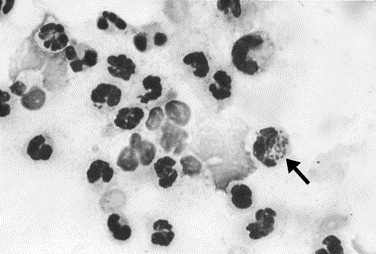
Canine septic arthritis. Neutrophils are increased in the fluid. Degenerate changes are evident in neutrophils and consist of vacuolated cytoplasms and indistinct cell margins. Amorphous round structures are disintegrated cell nuclei. Rod-shaped bacteria (arrow) are located within neutrophils (Wright stain, original magnification ×1000).
Fig. 8.
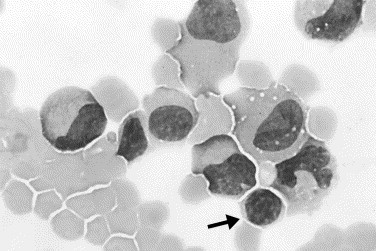
Joint effusion caused by trauma. A group of leukocytes at the edge of the smear is surrounded by red blood cells. Nucleated cells are small lymphocytes (arrow), monocytes, and macrophages (Wright stain, original magnification ×1000).
Fig. 9.
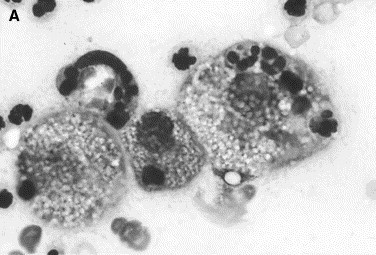
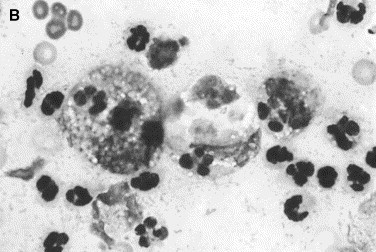
Canine inflammatory arthritis. Nucleated cell count is increased. Neutrophils, disintegrated nuclei, and large foamy macrophages are present. (A) The latter contain phagocytosed cells and cell nuclei. (B) The macrophage in the center has phagocytosed red blood cells in addition to some leukocytes (Wright stain, original magnification ×1000).
Cells that are seen infrequently but provide additional information include multinucleated giant cells, osteoclasts, and a variety of infectious agents [16], [36]. Multinucleated giant cells are derived from the fusion of macrophages and have multiple round to oval nuclei in a gray granular cytoplasm (Fig. 10 ). These cells may also phagocytose foreign material, fungi, or protozoa. Osteoclasts are 5 to 10 times the size of a neutrophil and have an irregular cell margin; abundant fine, granular, light blue-gray cytoplasm as well as several round nuclei that contain singular nucleoli (Fig. 11 ). These cells are seen rarely in joint fluid, but when present, they indicate cartilage damage with exposure of bone.
Fig. 10.
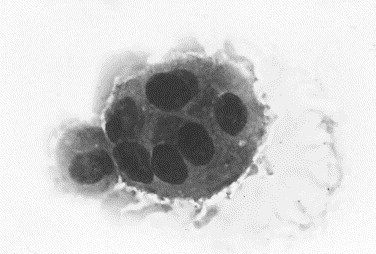
Canine inflammatory arthritis. This fluid contained numerous neutrophils and macrophages. At the periphery of the slide, an inflammatory giant cell was observed that had multiple nuclei and cytoplasmic features similar to a macrophage (left). These cells in joint fluid are usually observed with chronic inflammatory arthropathies (Wright stain, original magnification ×1000).
Fig. 11.
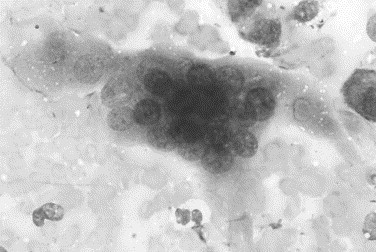
Canine joint fluid. The large multinucleate cell with fine granular cytoplasm and irregular cell margins is an osteoclast that is surrounded by red blood cells. This cell is seen rarely in joint fluid but usually indicates erosion of cartilage with exposure of bone (Wright stain, original magnification ×1000).
Synovial fluids from animals with immune-mediated arthritis have an increased TNCC with a neutrophilic or mixed inflammatory cell response. On rare occasions, cells are noted that confirm the presence of an immune-mediated disease [37]. Neutrophils containing small, round, purple, variably sized cytoplasmic granules are noted. The granules are easily confused with phagocytosed coccoid bacteria, but the granule size is too variable for bacterial organisms (Fig. 12 ). These cells have been referred to as ragocytes, and the granules represent phagocytosed droplets of nucleoprotein. Lupus erythematosus (LE) cells are another cell type that is indicative of immune-mediated arthritis [38]. These cells are detected rarely, even in those animals with positive antinuclear antibody titers and clinical signs of disease. LE cells are neutrophils that have phagocytosed bare nuclei. The neutrophil nucleus is marginated around a round, homogeneous, purple mass of nuclear material (Fig. 13 ). In some preparations of joint fluid, neutrophils are gathered around a central mass of nuclear material, which is a signal to search carefully for the typical LE cell phenomenon.
Fig. 12.
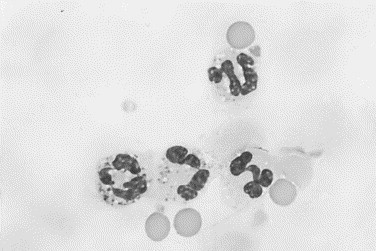
Canine immune-mediated arthritis. The neutrophil in the lower left contains small round cytoplasmic granules that vary in size. These inclusions are phagocytosed droplets of nucleoprotein and are seen occasionally in immune-mediated joint disease. Neutrophils with these inclusions have been referred to as ragocytes (Wright stain, original magnification ×1000).
Fig. 13.
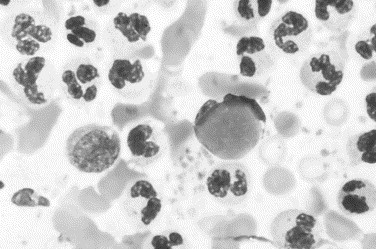
Canine immune-mediated arthritis. Neutrophils are numerous in a background that contains red blood cells and platelets. The latter indicate hemorrhage during arthrocentesis. The neutrophil in the center is a lupus erythematosus cell and contain a round homogeneous intracytoplasmic inclusion that represents phagocytosed nucleoprotein (Wright stain, original magnification ×1000).
Chemical assays
Most of the chemistry tests on synovial fluid produce results that support the cytologic and clinical assessment rather than providing new or discriminating information. Tests frequently included in synovial fluid analysis include measurement of total protein and mucin clot formation.
Total protein content is best measured by a quantitative biochemical method. Most laboratories estimate total protein by refractometry, however, and use a published reference range of 1.8 to 4.8 g/dL [6], [9]. In our laboratory, we have found that most normal joints fall in the range of 1.5 to 3.0 g/dL. Results by this method are not true indicators of protein content and are affected by other solutes. False increases in total protein can be caused by excessive EDTA relative to the amount of sample (short-filled EDTA tube) or by intra-articular injection of drugs. Total protein values are expected to increase with inflammation and the exudation of plasma proteins into the joint. Decreased protein values occur with transudation and after joint lavage with saline or lactated Ringer's solution.
The mucin clot test is a semiquantitative indicator of the amount of hyaluronic acid content based on acid precipitation. Synovial fluid collected in a plain tube or heparin tube is preferred, because the test is inhibited by the presence of EDTA. One part of synovial fluid is added to four parts of 2.5% glacial acetic acid in a test tube (Fig. 14 ). The precipitate formed is graded according to the following scale: good is indicated by a tight ropy clot in a clear solution, fair is indicated by a soft clot with a turbid solution, poor is indicated by a friable clot in a cloudy solution, and very poor is indicated by flocculent material in a cloudy solution [9]. If a small volume of fluid is collected, hyaluronic acid content can also be assessed by the viscosity of the fluid noted during collection and the density of the background staining observed microscopically.
Fig. 14.
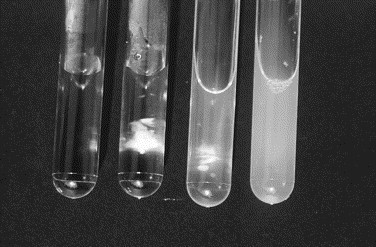
Mucin clot test. Hyaluronic acid (HA) content of synovial fluid can be measured semiquantitatively. Drops of synovial fluid are placed in 2.5% acetic acid solution. Tubes (left to right) contain acetic acid solution, tight ropy clot indicating normal HA content, small clot with turbid supernatant indicating reduced HA content, and turbid supernatant with no evidence of clot indicating extremely low HA content.
Synovial fluid pH can be measured with narrow-range pH paper. Measurement of pH must be done immediately after collection and cannot be done on EDTA samples. The reference range for synovial pH is 7.2 to 7.4. The pH decreases to values less than 6.9 within 12 to 24 hours after the initiation of joint sepsis in horses [43]. The usefulness of pH values in the differential diagnosis of canine and feline joint disease is uncertain without further clinical assessment.
Measurements of enzymes, glucose, urea, and electrolytes in synovial fluid have been reported in the experimental literature, but their value in clinical problem solving has not been established.
Microbial culture
Joint sepsis and immune-mediated arthritis can produce similar changes in the TNCC and cellular response. Therefore, we often rely on the results of bacterial cultures to make this differentiation. Samples for culture should not be collected in EDTA because it inhibits bacteria growth. Synovial fluid in plain tubes is best suited for microbiologic assays. If anaerobic bacteria are suspected, the sample must be protected from room air by collection into anaerobic transport media. The laboratory should be alerted if Mycoplasma is suspected, because these organisms grow better on specialized media.
Clinical experience and experimental studies indicate that direct culture of joint fluid on blood agar produces false-negative results in 50% to 70% of cases of septic arthritis. Studies have revealed that inoculating a pediatric blood culture bottle with synovial fluid, incubating for 24 hours, and then streaking the blood culture medium on appropriate plate media can significantly reduce false-negative culture results [39]. The liquid blood culture media prevents coagulation of the fluid, dilutes inhibitors, inactivates aminoglycoside antibiotics, and limits the in vitro phagocytosis of bacteria by leukocytes. Incubation of synovial cultures in blood culture medium has significantly enhanced the recovery of organisms from septic joints, which provides for an accurate diagnosis and correct therapy.
Laboratory abnormalities in joint disease
Noninflammatory arthritides
Degenerative joint disease is the classic example of a noninflammatory arthritis. Other conditions that may present with noninflammatory synovial fluid include traumatic joint disorders, hemarthrosis, neoplastic conditions involving the joint space, and arthropathies associated with viral infections in cats. Although classified as noninflammatory, these disorders are often characterized by a low-grade mononuclear inflammatory response within the synovium.
The results of synovial fluid analysis in these conditions may be normal or may reveal minor alterations. As a result of vasodilation, synovial fluid volume and total protein content may be mildly increased. The dilution of hyaluronic acid is mild, and viscosity is normal or slightly reduced. The mucin clot test remains normal or fair, because hyaluronic acid dilution or degradation is minimal. Synovial fluid color may vary from clear, straw colored, bloody, or xanthochromic depending on the extent and duration of intra-articular hemorrhage.
TNCCs may be normal to mildly elevated but are rarely greater than 5000 cells per milliliter [6]. Cytologic evaluation of the smear reveals a predominance of mononuclear cells with normal to slightly increased neutrophils (Table 1 ). The proportion of neutrophils should always be evaluated in light of the amount of blood present in the specimen. Mononuclear cells may be enlarged with an abundant foamy vacuolated cytoplasm that indicates increased phagocytic activity (Fig. 15 ) [33], [36]. With traumatic or coagulopathic hemarthrosis, phagocytic cells may contain erythrocytes or hemosiderin [33], [36]. All patients with multiple hemarthroses should be evaluated for a coagulopathy [6]. The presence of cartilage fragments, chondrocytes, and osteoclasts has been recognized as an indicator of severe cartilage damage in horses, with similar findings suggested in small animals [40]. Definitive identification of these components may require special stains.
Table 1.
Synovial fluid results in joint diseases of the dog and cat
| Differential (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Volume (mL) | Color | Clarity | Viscosity | Total protein (g/dL) | Mucin clot | TNCC (cells/μL) | Mono | Neut | Comments | Reference |
| Normal | 0.24 | Colorless to straw | Clear | High | <2.5 | Good | ≤3000 | ≥90 | <10 | [32] | |
| Noninflammatory | |||||||||||
| Degenerative joint disease | N to ↑ | Colorless to yellow | Clear | N to ↓ | <2.5 | Good to fair | ≤5000 | ≥90 | <10 | Macrophages may be vacuolated | [44] |
| Trauma | N to ↑ | Colorless, yellow bloody | Clear to hazy | ↓ | NR | Good | 2500–3000 | ≥90 | <10 | Neutrophil numbers depend on amount of hemorrhage present | [33] |
| Inflammatory | |||||||||||
| Infectious | |||||||||||
| Bacterial (rods/cocci) | N to ↑ | Yellow, bloody, or yellowish green | Cloudy to opaque | ↓ | >2.5 | Fair to very poor | 15,000– 267,000 | 5–23 | 77–95 | Neutrophils may be degenerate or nondegenerate; bacteria may be seen in neutrophils | [2], [33], [44] |
| Borellia burgdorferi (Lyme) | N to ↑ | Yellow to bloody | Cloudy | ↓ | >2.5 | Fair to poor | 46,300 (mean) | 15 (mean %) | 85 | Spirochetes rarely seen in synovial fluid by dark field microscopy | [14] |
| Ehrlichia sp | N to ↑ | Yellow to bloody | Cloudy | ↓ | >2.5 | Fair to poor | 38,800–50,000 | 20–40 | 60–80 | Ehrlichia morulae may be found in a small percentage of neutrophils | [12], [13] |
| Mycoplasma sp | N to ↑ | Yellow to bloody | Cloudy | ↓ | >2.5 | Fair to poor | Increased | Lymphocytic or neutrophilic | Requires special culture techniques | [15] | |
| Immune mediated | |||||||||||
| Idiopathic polyarthritis | N to ↑ | Yellow to bloody | Hazy to cloud | ↓ | >2.5 | Fair to poor | Increased | ≤10 | >90 | [6] | |
| Systemic lupus erythematosus | N to ↑ | Yellow to bloody | Hazy to cloudy | ↓ | >2.5 | Good to poor | 6200–371,000 | 7–85 | 15–93 | May see lupus erythematosus cell, ragocytes, Tart cells; ± ANA-positive | [5] |
| Canine rheumatoid arthritis | N to ↑ | Yellow to bloody | Hazy to cloudy | ↓ | >2.5 | Fair to poor | 2900–38,800 | Mononuclear or neutrophilic | May see ragocytes: ± RF - positive; | [31] | |
| Feline progressive polyarthritis | N to ↑ | Yellow to bloody | Hazy to cloudy | ↓ | >2.5 | Poor | 4000–70,000 | 1–75 | 25–99 | Erosive or proliferative forms | [19], [48] |
| Lymphoplasmacytic polyarthritis |
N to ↑ |
Yellow |
Hazy to cloudy |
↓ |
>2.5 |
NR |
5000–20,000 |
Mostly lymphocytic |
Causes stifle laxity; often mistaken for cranial cruciate rupture |
[25] |
|
Abbreviations: ANA, antinuclear antibodies; Mono, mononuclear cells; N, normal; Neut, neutrophils; NR, not reported; RF, rheumatoid factor; TNCC, total nucleated cell count; ↑, increased; ↓, decreased.
Fig. 15.
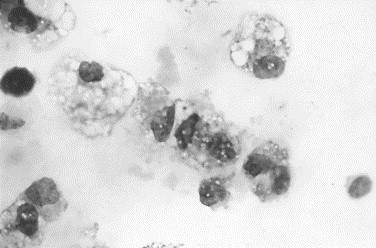
Canine degenerative joint disease. Most of the cells are large macrophages with granular vacuolated cytoplasms, suggesting that these cells are activated (Wright stain, original magnification ×1000).
A diagnosis of osteoarthritis is confirmed by finding typical radiographic changes in conjunction with characteristic physical findings and synovial fluid analysis. By the time these changes are seen, however, the condition is well established and cartilage damage is irreversible. Earlier recognition of a degenerative process can enhance our ability to treat, monitor, and assess the effect of therapy in these patients [41]. Potential markers of early osteoarthritis in synovial fluid may be related to the mechanism of cartilage damage or to the increased metabolism of cartilage components. Markers may be unique to the disorder or may be detected in greater quantities in the presence of disease [42]. MMP-3, a matrix metalloproteinase, is released from chondrocytes in association with osteoarthritis. It can digest several components of articular cartilage and is likely responsible for much of the damage seen in degenerative joint disease. An increase in the concentration of MMP-3 in synovial fluid has been well documented by several investigators and holds promise as an early marker of osteoarthritis [42]. Some investigators have found an increase in KS concentrations in the synovial fluid of dogs in models of osteoarthritis and believe it is caused by increased proteoglycan catabolism associated with this condition [42]. In contrast, others detected a decrease in KS in naturally occurring osteoarthritis and cranial cruciate ligament rupture [42]. They concluded that the release of immature proteoglycan from cartilage undergoing catabolism was deficient in KS. An altered form of CS was identified in the synovial fluid of dogs that underwent experimental cranial cruciate transection [42]. The authors concluded that this altered CS might be an indicator of early cartilage degradation. Studies using models of osteoarthritis that did not involve arthrotomies did not find significant elevations in synovial CS [42]. These and other markers have been characterized, but their clinical applicability is limited at this time. Some of these marker assays may become available commercially if their clinical value can be established.
Inflammatory arthritides
The inflammatory arthritides include a wide variety of disorders that share the characteristic of a suppurative infiltrate in the synovium. Included in this category are infectious arthritis and the more common immune-mediated disorders. Infrequent sources of a suppurative response are joint crystals, neoplastic disease, and intra-articular injections [6], [28]. In all forms, activation of the complement cascade mediates much of the damage seen [6].
Because the pathophysiology of these disorders is similar, the changes in synovial fluid may be indistinguishable from one another. Vascular leakage secondary to the effect of inflammatory mediators causes an increase in the volume of synovial fluid with capsular distention. Elevations in total protein and the presence of fibrin and coagulation proteins may cause the fluid to clot if it is not placed into an anticoagulant tube [6]. The color of the synovial fluid may be yellow to orange and often appears cloudy because of an increased number of nucleated cells (see Fig. 2). In cases of severe sepsis, the fluid may be opaque and appear yellowish green because of large numbers of degenerate neutrophils. Viscosity is often decreased as a result of enzymatic breakdown of hyaluronic acid by degradative enzymes derived from inflammatory cells or bacteria. Results of the mucin clot test are poor to very poor for similar reasons (see Fig. 14). Decreased polymerization of hyaluronic acid also contributes to decreased mucin quality, especially in immune-mediated disorders. Mucin clots graded as fair can be seen with less severe disease.
The defining characteristic of inflammatory arthritis is an increase in TNCCs with a predominance of neutrophils. Traditionally, the highest cell counts are associated with septic causes; however, the frequency of extremely high counts in immune-mediated arthritides precludes the ability to diagnose one or the other based on cell counts alone. Additionally, chronic infectious processes may have less dramatic elevations in cell counts [2]. Cytologically, absolute neutrophil numbers are increased and often represent the greatest proportion of cells. Neutrophil morphology is generally well preserved in immune-mediated arthritic diseases (Fig. 16 ). Unfortunately, this is often true for infectious arthritis as well; thus, the absence of degenerative changes does not rule out an infectious etiology [4]. Synovial fluids with increased neutrophils should always be examined microscopically for microorganisms (see Fig. 7).
Fig. 16.
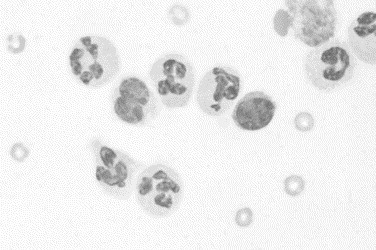
Immune-mediated arthritis in a dog. Nondegenerate neutrophils predominate, with fewer numbers of mononuclear cells. The background contains a few red blood cells and reduced granularity, indicating a reduction in hyaluronic acid content (Wright stain, original magnification ×1000).
The identification of microorganisms in cytologic preparations of synovial fluid can lead to a definitive diagnosis. Although the presence of phagocytosed bacteria confirms septic arthritis, bacteria are seen in roughly half of culture-positive synovial specimens [39], [47]. Begin a search for intracellular bacteria within clusters of neutrophils located in the body of the smear or at the feathered edge. Neutrophils that are well spread out or contain clear vacuoles are the best candidates. Ehrlichia morulae may be found in small numbers of neutrophils (1%) in the synovial fluid during acute stages of infection (Fig. 17 ) [12], [13]. Other organisms that may be found in synovial fluid include protozoa and fungal hyphae or yeast forms. Leishmania organisms have been seen within macrophages in otherwise normal synovial fluid [16]. Aspergillus sp and Blastomyces sp organisms have also been detected in synovial fluid (Fig. 18 ) [11], [44]. The cellular response is usually pyogranulomatous with fungal organisms and with bacterial L-forms (bacteria without cell walls) [8]. Microorganisms are rarely found in the synovial fluid of dogs with Lyme arthritis (Borrelia burgdorferi) or mycoplasmal arthritis [14], [15].
Fig. 17.
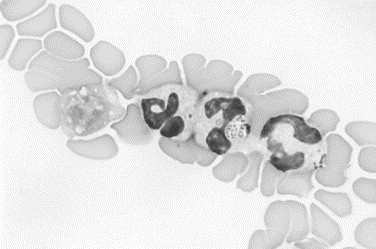
Canine joint fluid. Neutrophils at the feathered edge of the smear are surrounded by red blood cells. The round granular cytoplasmic inclusion in the central neutrophil is an Ehrlichia morula (Wright stain, original magnification ×1000).
Fig. 18.
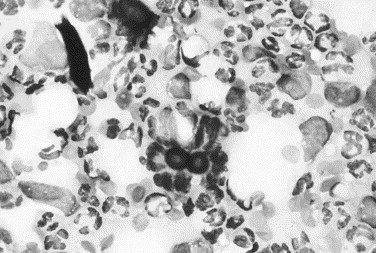
Canine inflammatory arthritis. The fluid from this joint was purulent. The smear reveals numerous neutrophils with a few monocytes and macrophages. A broad-based budding yeast is evident in the center and is consistent with Blastomyces infection (Wright stain, original magnification ×1000).
In small animals, immune-mediated disorders occur more frequently than infectious causes of arthritis. The presence or absence of radiographic evidence of cartilage erosion further subclassifies immune-mediated arthropathies. Although not pathognomonic, the presence of LE cells or ragocytes can support a diagnosis of an immune-mediated disorder [38]. LE cells, originally identified as an in vitro phenomenon, can occur in vivo. Tart cells can be confused with LE cells and are also neutrophils that have phagocytosed nuclear material. The nuclear material has not been acted on by antibodies, however, and retains the color and texture of normal chromatin [6], [9]. Ragocytes are found commonly in people with rheumatoid arthritis but occur infrequently in dogs that are similarly affected [9].
Joint diseases with variable synovial fluid results
Early or resolving inflammatory arthritides may present a diagnostic dilemma. Waxing and waning immune-mediated disorders may present with cell counts that are only mildly to moderately elevated with less than 50% neutrophils and may be mistaken for degenerative disorders. Repeat arthrocentesis may be needed to diagnose these cases during fulminant inflammation. Canine rheumatoid arthritis, an erosive immune-mediated disorder, may present with either a predominantly neutrophilic or predominantly mononuclear synovial fluid, making the diagnosis more difficult [5]. Lymphoplasmacytic synovitis, an immune-mediated condition characterized by synovial hyperplasia and nodular aggregates of lymphoid cells, exhibits a moderately elevated synovial fluid cell count (5000–20,000 cells per milliliter) with a predominance of small mononuclear cells, presumably lymphocytes [25]. Neutrophilic inflammation may be seen unexpectedly in cases of degenerative arthritis with as many as 12,000 cells per milliliter and up to 56% neutrophils [7]. Virally induced arthritis may present either as a mild or marked mononuclear effusion as in feline calicivirus or as a suppurative response [17], [18], [45].
Taking into account these difficulties, it is not always possible to differentiate between infectious and immune-mediated etiologies by synovial fluid evaluation alone. The history, physical examination, and serologic tests for infectious agents or immunologic disease may be necessary to separate the causes of suppurative arthritis [48]. The distribution of joint involvement may provide valuable information. Septic arthritis is usually monoarticular and most often affects the proximal joints (hip, shoulder, and stifle). Immune-mediated disorders are often pauciarticular (two to five joints) to polyarticular (more than five joints), with more severe disease in the distal joints (carpi and tarsi) [31]. Multiple joints may need to be sampled to demonstrate the polyarticular nature of the disorder. Monoarthritic immune-mediated disease occasionally affects only the elbow joint. Systemic infections with joint involvement usually present as polyarthritis, and the pathogenesis often has both an infectious and immune-mediated component. In addition, antigenic bacterial fragments may perpetuate inflammation initiated by a septic process in the absence of viable organisms [2]. Serologic tests for immune-mediated disorders (antinuclear antibody and rheumatoid factor) and infectious agents (Ehrlichia sp and B. burgdorferi) may assist in identification of the etiology.
Culture of synovial fluid is important in differentiating between infectious and immune-mediated arthritis. Attention should be paid to proper handling of the specimen and culturing techniques to capitalize on the probability of recovering the etiologic agent. Remember, however, that several infectious agents, such as Mycoplasma, bacterial L-forms, protozoa, or anaerobic bacteria, cannot be grown with routine culture procedures. The most common bacterial isolates from joint fluid are β-hemolytic streptococcus (Lancefield group C), staphylococci, hemolytic Escherichia coli, Pasteurella sp, and Erysipelothrix sp [2]. Less frequent isolates include Nocardia sp, Proteus sp, Pseudomonas sp, and anaerobic bacteria [22].
Uncommon causes of arthritis
Crystals injected into the synovial cavity induce a strong inflammatory response. This method is frequently used as an experimental model of suppurative arthritis. Gout, an inflammatory arthritis caused by intra-articular uric acid crystals in birds and human beings, is not recognized in cats and dogs. A few cases of pseudogout have been reported in dogs and are characterized by the accumulation of calcium pyrophosphate crystals in synovial tissue and the joint space [29]. Unlike uric acid crystals, calcium pyrophosphate crystals are radiopaque.
Neoplasms may arise in a joint or invade or metastasize to the synovial tissue. Primary tumors of the joint, such as synovial sarcomas, can invade the joint space, allowing for the identification of tumor cells in the synovial fluid (Fig. 19 ). Carcinomas can metastasize to single or multiple joints. Often, the detection of pleomorphic epithelial cells in the synovial fluid is the first indicator of carcinoma located elsewhere [26], [27], [28]. Neoplastic invasion into the synovial cavity usually induces a suppurative response secondary to necrosis and joint damage.
Fig. 19.
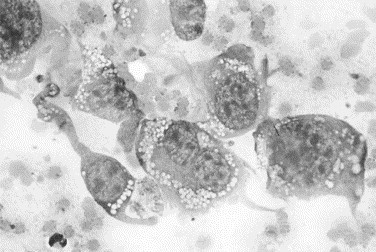
Canine joint fluid. This joint was hot, painful, and swollen, with radiographic evidence of osteolysis. The fluid is hemorrhagic and contains neutrophils and mononuclear cells. A second population of cells exhibiting marked variation in cell size and shape, variable nuclear-to-cytoplasmic ratios, variation in nuclear size, and extremely variable nucleolar size and shape is visible. These cells are consistent with a malignancy of connective tissue origin. The histologic diagnosis was synovial cell sarcoma (Wright stain, original magnification ×1000).
Summary
Canine and feline joint disease can be a primary disorder limited to joints or a manifestation of multisystemic disease. Collection and analysis of joint fluid provides valuable information for the diagnosis, prognosis, and treatment of diseases that affect the joint space. The cytologic recognition of the cellular components and infectious agents in synovial fluid categorizes the cell response and differentiates inflammatory and noninflammatory joint disorders. This information is supported by the cell counts, protein content, mucin clot test, bacterial culture, and serologic tests for infectious or immune-mediated disease. These results are integrated with the clinical history, physical examination, radiographic findings, and ancillary test results to arrive at a diagnosis and treatment plan.
References
- 1.Willard M. Fluid accumulation disorders. In: Willard M., Tvedten H., Turnwald G., editors. Small animal clinical diagnosis by laboratory methods. 1st edition. WB Saunders; Philadelphia: 1989. pp. 229–242. [Google Scholar]
- 2.Marchevsky A., Read R. Bacterial septic arthritis in 19 dogs. Aust Vet J. 1999;77:233–237. doi: 10.1111/j.1751-0813.1999.tb11708.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnston S. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997;27:699–734. doi: 10.1016/s0195-5616(97)50076-3. [DOI] [PubMed] [Google Scholar]
- 4.Beale B. Arthropathies. In: Bloomberg M.S., Dee J.F., Taylor R.A., editors. Canine sports medicine and surgery. 1st edition. WB Saunders; Philadelphia: 1998. pp. 210–222. [Google Scholar]
- 5.Pedersen N., Weisner K., Castles J. Noninfectious canine arthritis: the inflammatory, nonerosive arthritides. JAVMA. 1976;169:304–310. [PubMed] [Google Scholar]
- 6.Ellison R. The cytologic examination of synovial fluid. Semin Vet Med Surg (Small Anim) 1988;3:133–139. [PubMed] [Google Scholar]
- 7.Griffin D., Vasseur P. Synovial fluid analysis in dogs with cranial cruciate ligament rupture. J Am Anim Hosp Assoc. 1992;28:277–281. [Google Scholar]
- 8.Carro T., Pedersen N., Bellah J. Subcutaneous abscesses and arthritis caused by a probable bacterial L-form in cats. JAVMA. 1989;194:1583–1588. [PubMed] [Google Scholar]
- 9.Boon D. Synovial fluid analysis: a guide for small-animal practitioners. Vet Med. 1997;92:443–451. [Google Scholar]
- 10.Huss B., Collier L., Collins B. Polyarthropathy and chorioretinitis with retinal detachment in a dog with systemic histoplasmosis. J Am Anim Hosp Assoc. 1994;30:217–224. [Google Scholar]
- 11.Oxenford C., Middleton D. Osteomyelitis and arthritis associated with Aspergillus fumigatus in a dog. Aust Vet J. 1986;63:59–61. doi: 10.1111/j.1751-0813.1986.tb02925.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellah J., Shull R., Selcer Shull E. Ehrlichia canis-related polyarthritis in a dog. JAVMA. 1986;189:922–923. [PubMed] [Google Scholar]
- 13.Cowell R., Tyler R., Clinkenbeard K. Ehrlichiosis and polyarthritis in three dogs. JAVMA. 1988;192:1093–1095. [PubMed] [Google Scholar]
- 14.Kornblatt A., Urband P., Steere A. Arthritis caused by Borrelia burgdorferi in dogs. JAVMA. 1985;186:960–964. [PubMed] [Google Scholar]
- 15.Ernst S., Goggin J. What is your diagnosis? JAVMA. 1999;215:19–20. [PubMed] [Google Scholar]
- 16.Yamaguchi R., French T., Simpson C. Leishmania donovani in the synovial fluid of a dog with visceral leishmaniasis. J Am Anim Hosp Assoc. 1983;19:723–726. [Google Scholar]
- 17.Dawson S., Bennett D., Carter S. Acute arthritis of cats associated with feline calicivirus infection. Res Vet Sci. 1994;56:133–143. doi: 10.1016/0034-5288(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 18.Levy J., Marsh A. Isolation of calicivirus from the joint of a kitten with arthritis. JAVMA. 1992;201:753–755. [PubMed] [Google Scholar]
- 19.Pedersen N. Synovial fluid collection and analysis. Vet Clin North Am Small Anim Pract. 1978;8:495–499. doi: 10.1016/s0091-0279(78)50056-7. [DOI] [PubMed] [Google Scholar]
- 20.Woodard J., Riser W., Bloomberg M. Erosive polyarthritis in two Greyhounds. JAVMA. 1991;198:873–876. [PubMed] [Google Scholar]
- 21.Dougherty S., Center S., Shaw E. Juvenile-onset polyarthritis syndrome in Akitas. JAVMA. 1991;198:849–856. [PubMed] [Google Scholar]
- 22.Carr A. Infectious arthritis in dogs and cats. Vet Med. 1997;92:786–810. [Google Scholar]
- 23.Pedersen N., Pool R., O'Brien T. Feline chronic progressive polyarthritis. Am J Vet Res. 1980;41:522–535. [PubMed] [Google Scholar]
- 24.Giger U., Werner L., Millichamp N. Sulfadiazine-induced allergy in six Doberman Pinschers. JAVMA. 1985;186:479–484. [PubMed] [Google Scholar]
- 25.Pedersen N., Wind A., Morgan J. Joint diseases in dogs and cats. In: Ettinger, editor. The textbook of veterinary internal medicine. 3rd edition. WB Saunders; Philadelphia: 1989. pp. 2329–2377. [Google Scholar]
- 26.Lowseth L., Gillett N., Muggenburg B. What is your diagnosis? Vet Clin Pathol. 1989;18:88–89. doi: 10.1111/j.1939-165x.1989.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 27.Lowseth L., Herbert R., Muggenburg B. What is your diagnosis? Vet Clin Pathol. 1989;18:57. doi: 10.1111/j.1939-165x.1989.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 28.Meinkoth J., Rochat M., Cowell R. Metastatic carcinoma presenting as hind-limb lameness: diagnosis by synovial fluid cytology. J Am Anim Hosp Assoc. 1997;33:325–328. doi: 10.5326/15473317-33-4-325. [DOI] [PubMed] [Google Scholar]
- 29.Gibson J., Roenigk W. Pseudogout in a dog. JAVMA. 1972;161:912–915. [PubMed] [Google Scholar]
- 30.Hardy R., Wallace L. Arthrocentesis and synovial membrane biopsy. Vet Clin North Am Small Anim Pract. 1974;4:449–462. doi: 10.1016/s0091-0279(74)50044-9. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen N., Pool R., Castles J. Noninfectious canine arthritis: rheumatoid arthritis. JAVMA. 1976;169:295–303. [PubMed] [Google Scholar]
- 32.Sawyer D. Synovial fluid analysis of canine joints. JAVMA. 1963;143:609–612. [PubMed] [Google Scholar]
- 33.Fernandez F., Grindem C., Lipowitz A. Synovial fluid analysis: preparation of smears for cytologic examination of canine synovial fluid. J Am Anim Hosp Assoc. 1983;19:727–734. [Google Scholar]
- 34.Palmer D. Total leukocyte enumeration in pathologic synovial fluids. Am J Clin Pathol. 1968;49:812–814. doi: 10.1093/ajcp/49.6.812. [DOI] [PubMed] [Google Scholar]
- 35.Gibson N., Carmichael S., Li A. Value of direct smears of synovial fluid in the diagnosis of canine joint disease. Vet Rec. 1999;144:463–465. doi: 10.1136/vr.144.17.463. [DOI] [PubMed] [Google Scholar]
- 36.Kusba J., Lipowitz A., Wise M. Suspected villonodular synovitis in a dog. JAVMA. 1983;182:390–392. [PubMed] [Google Scholar]
- 37.Parry B. Synovial fluid. In: Cowell R., Tyler R., Meinkoth J., editors. Diagnostic cytology and hematology of the dog and cat. 2nd edition. Mosby; St. Louis: 1999. pp. 104–119. [Google Scholar]
- 38.Schalm O., Ling G. The L.E. phenomenon in the dog. Calif Vet. 1970;24:20–25. [Google Scholar]
- 39.Montgomery R., Long I., Milton J. Comparison of aerobic culturette, synovial membrane biopsy, and blood culture medium in detection of canine bacterial arthritis. Vet Surg. 1989;18:300–303. doi: 10.1111/j.1532-950x.1989.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 40.Freeman K., Todhunter R., Lust G. Cytology of polychrome-stained equine synovial fluid. Acta Cytol. 1991;35:512–520. [PubMed] [Google Scholar]
- 41.Rorvik A., Grondahl A. Markers of osteoarthritis: a review of the literature. Vet Surg. 1995;24:255–262. doi: 10.1111/j.1532-950x.1995.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 42.Fox D., Cook J. Synovial fluid markers of osteoarthritis in dogs. JAVMA. 2001;219:756–761. doi: 10.2460/javma.2001.219.756. [DOI] [PubMed] [Google Scholar]
- 43.Tulamo R., Bramlage L., Gabel A. Sequential clinical and synovial fluid changes associated with acute infectious arthritis in the horse. Equine Vet J. 1989;21:325–331. doi: 10.1111/j.2042-3306.1989.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 44.Bloomberg M., Ackerman N., Buergelt C. Cryptococcal arthritis and osteomyelitis in a dog. Compend Contin Educ Pract Vet. 1983;5:609–616. [Google Scholar]
- 45.Bennett D., Gaskell R., Mills A. Detection of feline calicivirus antigens in the joints of infected cats. Vet Rec. 1989;124:329–332. doi: 10.1136/vr.124.13.329. [DOI] [PubMed] [Google Scholar]
- 46.Atilola M., Lumsden J., Rooke F. A comparison of manual and electronic counting for total nucleated cell counts on synovial fluid from canine stifle joints. Can J Vet Res. 1986;50:282–284. [PMC free article] [PubMed] [Google Scholar]
- 47.Madison J., Sommer M., Spenser P. Relations among synovial membrane histopathologic findings, synovial fluid cytologic findings, and bacterial culture results in horses with suspected infectious arthritis: 64 cases (1979–1987) JAVMA. 1991;198:1655–1661. [PubMed] [Google Scholar]
- 48.Wilkinson G., Robins G. Polyarthritis in a young cat. J Small Anim Pract. 1979;20:293–297. doi: 10.1111/j.1748-5827.1979.tb06723.x. [DOI] [PubMed] [Google Scholar]


