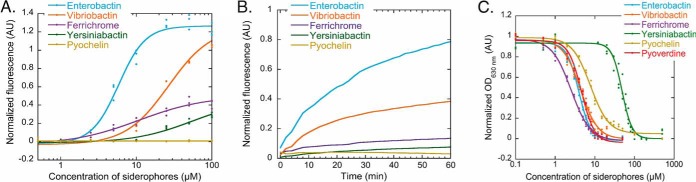
Fig. 3.
A, Ability of various siderophores to steal iron from PVD-Fe complexes. For these titration assays, PVD-Fe at 10 μm in 100 mm HEPES pH 7.4 was incubated with various concentrations of ENT, VIB, FERRI, YER and PCH (until equilibrium was reached, 48 h) as described in Materials and Methods. Apo PVD is fluorescent and PVD-Fe is not. Apo PVD formation was thus followed by monitoring its fluorescent at 447 nm (excitation at 400 nm). The data were normalized using (FMEASURED - FPVD-Fe)/(FPVD - FPVD-Fe), FMEASURED for the fluorescence measured for each experimental condition, FPVD-Fe for the fluorescence of 10 μm PVD-Fe, and FPVD for the fluorescence of 10 μm PVD. B, Kinetics of PVD-Fe dissociation in the presence of various siderophores. PVD-Fe (10 μm in 100 mm HEPES buffer pH 7.4) was incubated with 100 μm ENT, VIB, FERRI, YER, or PCH as described in Materials and Methods. The kinetics of apo PVD formation were followed by monitoring the fluorescence emission at 447 nm (excitation at 400 nm). C, Ability of various siderophores to steal iron from the CAS-Fe complex. CAS-Fe at 7.5 μm was incubated with various concentrations of ENT, VIB, FERRI, YER, PCH, or PVD as described in Materials and Methods. CAS-Fe dissociation was followed by monitoring the absorbance at 630 nm. The data were normalized using (AMEASURED - AapoCAS)/(AapoCAS - ACAS-Fe), AMEASURED for the absorbance measured for each experimental condition, AapoCAS for the absorbance of CAS without iron and ACAS-Fe for the absorbance of CAS loaded with iron.