Fig. 2. MII spindle rotation requires Arp2/3 complex, myosin-II, and dynamic F-actin network.
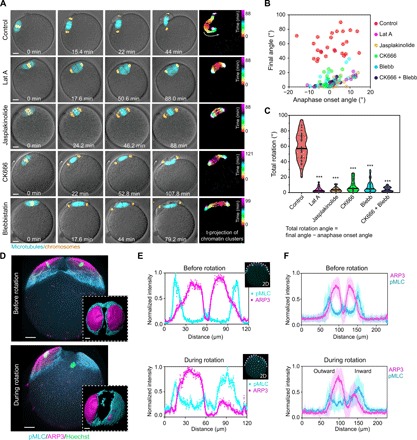
(A) Montages from live imaging showing that inhibition of actin polymerization (Lat A treatment), actin depolymerization (jasplakinolide treatment), Arp2/3 complex (CK666 treatment), or myosin-II (blebbistatin treatment) prevented spindle rotation. Time projections of chromatin clusters are shown on the right as in Fig. 1C. (B and C) Quantification of spindle angle at anaphase onset and final angle (B) of the spindle after various treatments as indicated. Total rotation angle (C) equals the final spindle angle minus that at anaphase onset. ***P < 0.001. (D) 3D projected images of immunofluorescence staining showing that ARP3 and active myosin-II [phosphorylated myosin light chain (pMLC)] changed from a symmetric distribution to an asymmetric distribution on the cortex overlying chromatin clusters during spindle rotation. Top views of 3D reconstructed ARP3 and myosin-II are shown in the bottom insets. (E) Fluorescence intensity profiles of ARP3 and pMLC in a middle optical section across the spindle proximal pole in the oocyte from (D), with colored curves displaying smoothened data. (F) Line profiles of ARP3 and pMLC fluorescence intensity from an optical section parallel to the spindle and cutting across the spindle proximal cortex in oocytes prerotation (averaged for 11 oocytes, means ± SD) and during rotation (averaged for 13 oocytes, means ± SD). Scale bars, 10 μm (for all images).
