Fig. 1. Fundamental contractile parameters of WT (black) and R663H (dark red) human β-cardiac sS1.
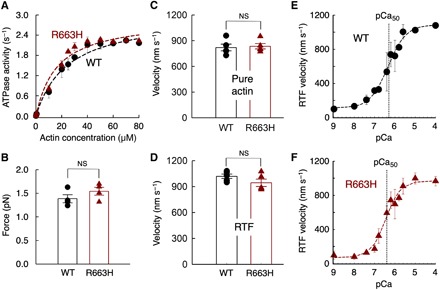
(A) Actin-activated ATPase data for WT and R663H sS1. Fitting the traces yielded kcat values of 3.0 ± 0.2 and 2.9 ± 0.1 s−1 for WT and R663H sS1, respectively. Dashed lines are the fitted lines. Representative data (average of two experiments from single preparations of both proteins) are shown. (B) Intrinsic force measurements of WT (black) and R663H (dark red) human β-cardiac sS1 using an optical trap. The individual force values were averaged from force measurements of four molecules of each motor. (C) Comparison of in vitro motility of actin filaments for WT (black) and R663H (dark red) human β-cardiac sS1 (five different experiments from five independent protein preparations for each of WT and R663H sS1). (D) Comparison of in vitro motility of regulated thin filaments at pCa = 4.0 for WT (black) and R663H (dark red) human β-cardiac sS1 (five different experiments from four independent protein preparations for each of WT and R663H sS1). (E) Ca2+ sensitivity measurements for WT human β-cardiac sS1 (four different experiments were averaged). (F) Ca2+ sensitivity measurements for R663H human β-cardiac sS1 (four different experiments were averaged). The data for (E) and (F) were fitted (dashed lines) to the Hill equation to estimate the pCa50. When each of the four individual experiments was fitted to the Hill equation separately, mean pCa values of 6.3 ± 0.2 and 6.5 ± 0.2 were obtained for WT and R663H, respectively. For all panels, error bars denote SEM. NS, not significant, P > 0.05. (B) P = 0.24; (C) P = 0.78; (D) P = 0.18; (E and F) P = 0.46 (four individual experiments were used for calculation).
