Fig. 2. The affinity of WT and R663H human ß-cardiac sS1 binding to proximal S2.
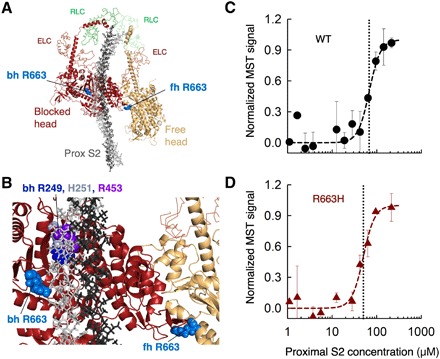
(A) Structural model of the IHM state for human β-cardiac myosin [human sequestered state model; from Robert-Paganin et al. (14)]. The S1 portion of the heavy chain is shown in cartoon mode (PyMOL), the light chains are in ribbon mode, and the proximal S2 is in stick mode. The positions of the blocked head (bh) and free head (fh) Arg663 residues are shown as spheres in light blue. (B) Blowup of the IHM model showing the relationships between the Arg663 residues and the proximal S2 position and the head-head interaction zone. Bh R249 (blue), H251 (gray), and R453 (purple) are shown for reference. (C) MST binding data for WT human ß-cardiac sS1 and proximal S2 in 100 mM KCl. (D) MST binding data for R663H human ß-cardiac sS1 and proximal S2in 100 mM KCl. Data in (C) and (D) are representative data from two measurements from a single set of protein preparations.
