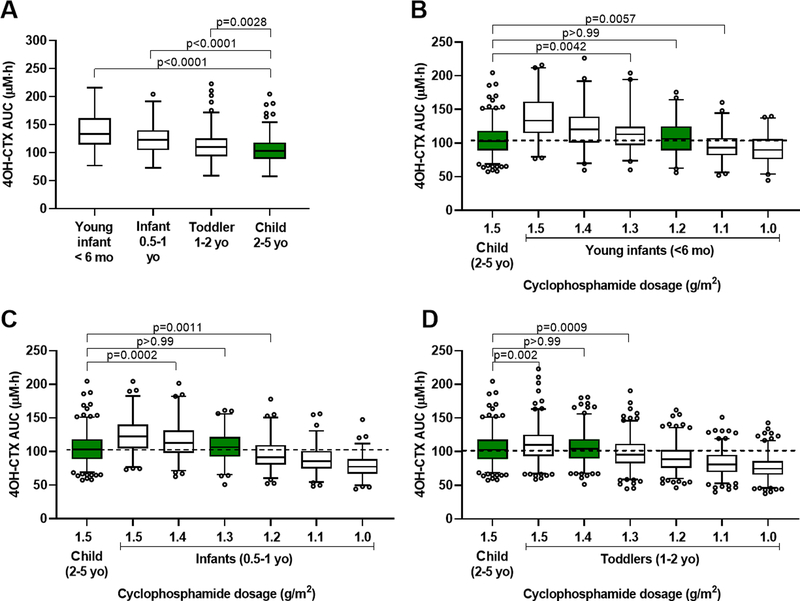
Figure 4.
Age-based cyclophosphamide dosing adjustment simulations to attain similar 4OH-CTX plasma exposure (AUC0–24h) across pediatrics. (A) 4OH-CTX exposure ranges (AUC0–24h) after a 1-hour infusion of cyclophosphamide (1.5 g/m2) in young infants (< 6 months), infants (0.5–1 year), toddlers (1–2 years), and young children (2–5 years). (B) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in young infants vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. (C) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in infants vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. (D) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in toddlers vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. P values results from Dunnett’s multiple comparisons tests.