Table 1.
Optimization of Bicyclization Reaction Conditions
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | silane | Y | Solventb | yield (%)c |
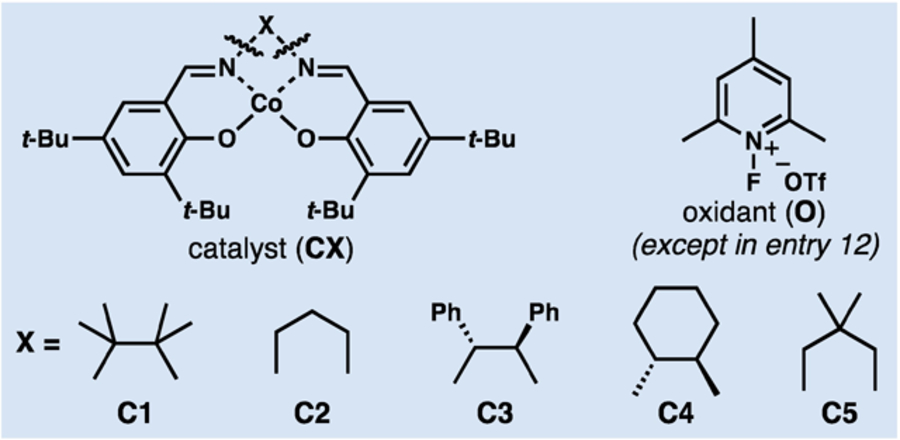
| 1 | C1 | PhSiH3 | 3.0 | Me2CO | 40 |
| 2 | C1 | PhSiH3 | 0.5 | Me2CO | <5d |
| 3 | C1 | Ph(i-PrO)SiH2 | 3.0 | Me2CO | 45 |
| 4 | C1 | TMDSO | 3.0 | Me2CO | 42 |
| 5 | C1 | PhSiH3 | 3.0 | i-PrOH | 0 |
| 6 | C1 | TMDSO | 2.0 | PhCF3 | 0 |
| 7 | C1 | TMDSO | 3.0 | CH2Cl2 | <5 |
| 8 | C1 | TMDSO | 3.0 | i-PrOH | <5 |
| 9 | C1 | TMDSO | 3.0 | HFIP | 87 |
| 10 | C2 | TMDSO | 3.0 | HFIP | 31d |
| 11 | C3 | TMDSO | 3.0 | HFIP | <5 |
| 12 | C1 | TMDSO | 0 | HFIP | <5d |
| 13e | C1 | TMDSO | O2 | HFIP | <5 |
| 14 | – | TMDSO | 3.0 | HFIP | 0 |
 | |||||
All reactions were carried out using 5a (0.02 mmol), oxidant, catalyst (0.002 mmol), silane (0.07 mmol), in solvent (0.5 M) at ambient temperature (rt) for 4h under Ar.
Reaction mixtures were purged with Ar for 10 min. before the addition of silane.
For entries 1, 3, 4, 8, 9 isolated yields are reported, for other entries yields were estimated via 1H NMR spectroscopic analysis.
Incomplete conversion was observed.
Reaction was run for 12h. TBS: terti-butyldimethylsilyl; TMDSO: tetramethyldisiloxane; HFIP: 1,1,1,3,3,3-hexafluoroisopropanol
