Table 1.
Biochemical profile of compounds 1–4[a].
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cpd | R | R1 | R2 | R3 | R4 | R5 | R6 | LSD1, IC50 (μM) | % inhibition at 100 μM | |
| MAO A | MAO B | |||||||||
| 1a[b] |  |
0.019 ± 0.004 | 97% | NI[c] | ||||||
| 1b |  |
0.106 ± 0.020 | 96% | 37% | ||||||
| 1c |  |
0.024 ± 0.004 | 96% | 50% | ||||||
| 1d |  |
0.029 ± 0.005 | 95% | NI | ||||||
| 1e |  |
0.051 ± 0.008 | 2% | 8% | ||||||
| 1f |  |
0.054 ± 0.010 | 96% | 33% | ||||||
| 1g |  |
0.038 ± 0.009 | 98% | 48% | ||||||
| 1h | 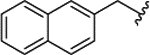 |
0.043 ± 0.010 | 89% | 17% | ||||||
| 2a[d] | 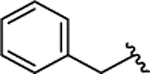 |
H | 0.149 ± 0.028 | 94% | 1% | |||||
| 2b[e] |
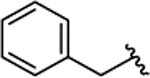 meta |
H | 18.1 ± 2.9 | |||||||
| 2c |  |
H | 0.459 ± 0.090 | |||||||
| 2d | 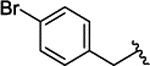 |
H | 0.230 ± 0.042 | |||||||
| 2e | 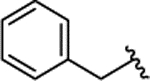 |
Br | 0.080 ± 0.026 | 71% | 29% | |||||
| 2f |  |
H | 0.236 ± 0.039 | |||||||
| 2g |  |
H | 0.130 ± 0.025 | 87% | 42% | |||||
| 2h | 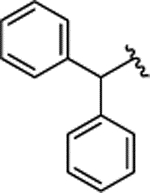 |
H | 0.280 ± 0.052 | |||||||
| 2i | 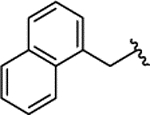 |
H | 0.173 ± 0.028 | |||||||
| 2j | 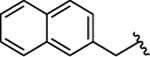 |
H | 0.767 ± 0.104 | |||||||
| 2k |  |
H | 0.190 ± 0.030 | |||||||
| 2l | 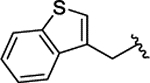 |
H | 0.083 ± 0.012 | |||||||
| 2m | 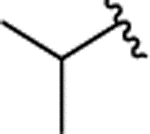 |
H | 0.400 ± 0.072 | |||||||
| 2n | 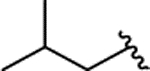 |
H | 0.236 ± 0.040 | |||||||
| 2o | 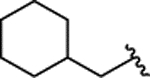 |
H | 0.581 ± 0.071 | |||||||
| 3a | H | H |  |
H | 0.090 ± 0.012 | 37% | 8% | |||
| 3b[f] | H | 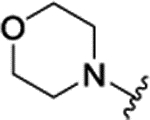 |
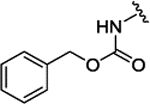 |
H | 0.075 ± 0.011 | 76% | 78% | |||
| 3c | H | 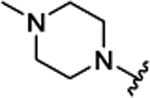 |
 |
H | 0.089 ± 0.010 | 69% | 55% | |||
| 3d[f] | H | 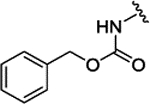 |
H | H | 0.043 ± 0.010 | 46% | NI | |||
| 3e[f] | H |  |
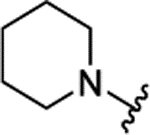 |
H | 0.307 ± 0.069 | |||||
| 3f[f] | H | 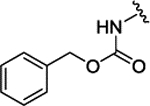 |
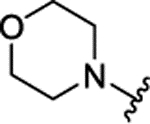 |
H | 0.061 ± 0.011 | 21% | 17% | |||
| 3g[f] | H |  |
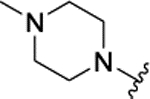 |
H | 0.089 ± 0.020 | 5% | 13% | |||
| 3h | H |  |
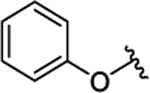 |
H | 0.143 ± 0.028 | |||||
| 3i | H | 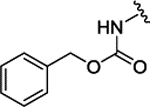 |
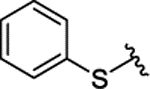 |
H | 0.073 ± 0.011 | |||||
| 3j |  |
H | H | H | 0.146 ± 0.022 | 72% | 17% | |||
| 3k | 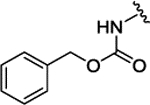 |
H | Br | H | 0.159 ± 0.034 | 94% | NI | |||
| 3l | 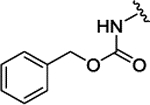 |
H | 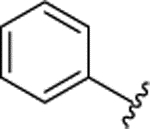 |
H | 0.135 ± 0.029 | |||||
| 3m |  |
H | H | 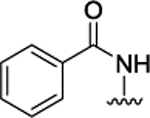 |
3.9 ± 0.2 | |||||
| 3n | 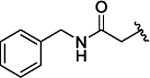 |
H | H | 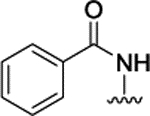 |
1.7 ± 0.05 | |||||
| 4a | 10.8 ± 1.1 | |||||||||
| 4b | 56.2 ± 7.8 | |||||||||
| 4c | NI | |||||||||
| 4d | NI | |||||||||
| 4e | NI | |||||||||
| 4f | NI | |||||||||
| 4g | NI | |||||||||
| 4h | NI | |||||||||
| 4i | NI | |||||||||
| TCP | 11.2 ± 1.8 | 79% | 85% | |||||||
Data represent mean values of at least two separate experiments in duplicate.
Ref. [13a], added for comparison.
NI, no inhibition.
Reff. [13b, 19], added for comparison; compounds 2a,c-o have the Z-aminoacylamino substituent inserted at the C4 position of the TCP phenyl ring.
C3-substituted regioisomer of 2a.
Ref. [14], added for comparison.
