Abstract
The link between psoriasis and obstructive sleep apnea (OSA) has not been confirmed. We aimed to investigate the relationship between psoriasis and OSA. We conducted a systematic review and meta-analysis of case-control, cross-sectional, and cohort studies on the association between psoriasis and OSA. We searched MEDLINE and Embase for relevant studies on May 11, 2019. The Newcastle-Ottawa Scale was used to evaluate the risk of bias of included studies. We performed random-effects model meta-analysis to calculate pooled odds ratio (ORs) with 95% confidence intervals (CIs) for case-control and cross-sectional studies as well as pooled incidence rate ratio (IRR) with 95% CIs for cohort studies in association between psoriasis and OSA. A total of 4 case-control or cross-sectional studies and 3 cohort studies with a total of 5,840,495 subjects were included. We identified a significantly increased odds for OSA in psoriasis patients (pooled OR 2.60; 95% CI 1.07–6.32), and significantly increased risk for psoriasis in OSA patients (pooled IRR 2.52; 95% CI 1.89–3.36). In conclusion, our study identified a bidirectional association between psoriasis and OSA. Sleep quality should be inquired in patients with psoriasis. Respirologist consultation or polysomnography may be indicated for those presenting with night snoring, recurrent awaking, and excessive daytime sleepiness.
Subject terms: Risk factors, Skin diseases
Introduction
Psoriasis is a chronic inflammatory skin disease with characteristic feature of sharply circumscribed erythematous plaques with silvery scales on the trunk and limbs1. The prevalence was estimated 0.5–11.4% in adults2. Psoriasis is considered as a multisystemic disease involving proinflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-173,4. Various comorbidities have been linked to psoriasis including cardiovascular disease, metabolic syndrome, chronic kidney disease, uveitis, thyroid diseases, vitiligo, and inflammatory bowel disease5–11.
Obstructive sleep apnea (OSA), also called obstructive sleep apnea-hypopnea syndrome, is a chronic disorder of intermittent upper airway collapse during sleep resulting in recurrent hypoxia12,13. OSA is characterized with night snoring, recurrent awaking, and excessive daytime sleepiness14. The gold standard test for diagnosing OSA is polysomnography15. The prevalence of OSA ranges from 3 to 17%12. Risk factors of OSA include obesity, aging, male gender, anatomical predisposition, and alcohol consumption12,13. OSA induces systemic inflammatory and increases the risk of hypertension, stroke, cardiovascular disease, and metabolic disorder, in particular of diabetes mellitus type 2 and metabolic syndrome regardless of the obesity15–17.
Psoriasis and OSA share a common pathogenesis of inflammatory and immune imbalance18,19. The association between psoriasis and OSA has been examined in many studies but the results were limited and inconsistent20–27. The objective of this study was to assess the evidence regarding the bidirectional association between psoriasis and OSA.
Methods
We conducted a systematic review and meta-analysis of observational studies (case-control, cross-sectional, and cohort studies) on the association between psoriasis and OSA. The reporting of this study followed the Meta-analysis of Observational studies in Epidemiology (MOOSE) guidelines28. We have registered the study protocol with PROSPERO (CRD42019129605; see https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=129605).
Literatures search
We searched the MEDLINE and Embase for relevant studies from inception to May 11, 2019. The search terms included psoriasis and sleep apnea and their synonyms. The search strategy is shown in the Supplement. No geographic or language limitations were imposed.
Study selection
The criteria for studies to be included were as follows: (1) observational studies investigating the association between psoriasis and OSA, including case-control, cross-sectional, and cohort studies; (2) research on human subjects; (3) the case group consisted of patients with psoriasis and the control group consisted of people without psoriasis in studies that examined the odds or risk of OSA in psoriasis patients; the study group was composed of patients with OSA and the control group was composed of people without OSA in studies that evaluated the odds or risk of psoriasis in OSA patients. We screened titles and abstracts for the initial study selection and obtained the full text of potentially eligible studies to confirm if they met our inclusion criteria. Two authors (T.G. and Y.F.) independently selected studies and disagreement was resolved by consulting the other author (C.C.).
Data extraction
We extracted the following data from the included studies: first author, year of publication, country of study, number and gender of study subjects, definition of the case group, definition of outcome, and risk estimates including hazard ratio (HR), incidence rate ratio (IRR), and odds ratio (OR) with 95% confidence interval (CI) on the association between psoriasis and OSA.
Risk of bias evaluation
The risk of bias of included studies was assessed by using the Newcastle-Ottawa Scale29. For case-control and cross-sectional studies, we examined the following eight domains: adequacy of case definition, representativeness of cases, selection of controls, definitions of controls, comparability of cases and controls, ascertainment of exposure, same method of ascertainment for cases and controls, and non-response rate. For cohort studies, we evaluated the following eight domains: representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, absence of outcome of interest at start of study, comparability of cohorts, assessment of outcome, follow-up duration, and adequacy of follow up of cohorts.
Statistical analysis
The Review Manage version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for conducting all analyses. We calculated the OR with 95% CI in included case-control and cross-sectional studies and IRR in included cohort studies. If OR was not reported in an included study, we calculated the crude OR based on published data for example the number of events in the case and control groups. The most fully adjusted OR, HR, or IRR were adopted if reported. We performed meta-analyses to evaluate the bidirectional association between psoriasis and OSA. For included case-control and cross-sectional studies, we calculated pooled OR to examine the association between the two diseases. For cohort studies, we treated HR as IRR and calculated the pooled IRR30. The statistical heterogeneity was assessed by the I2 statistic across the included studies. An I2 of >50% represents substantial heterogeneity31. We chose random-effects model for meta-analyses because clinical heterogeneity was anticipated. Therefore the DerSimonian and Laird method that takes between-study variability into account was used to obtain the pooled OR and IRR estimates32. Also, we conducted a sensitivity analysis after excluding studies that were rated with a high risk of bias.
Results
Characteristic of included studies
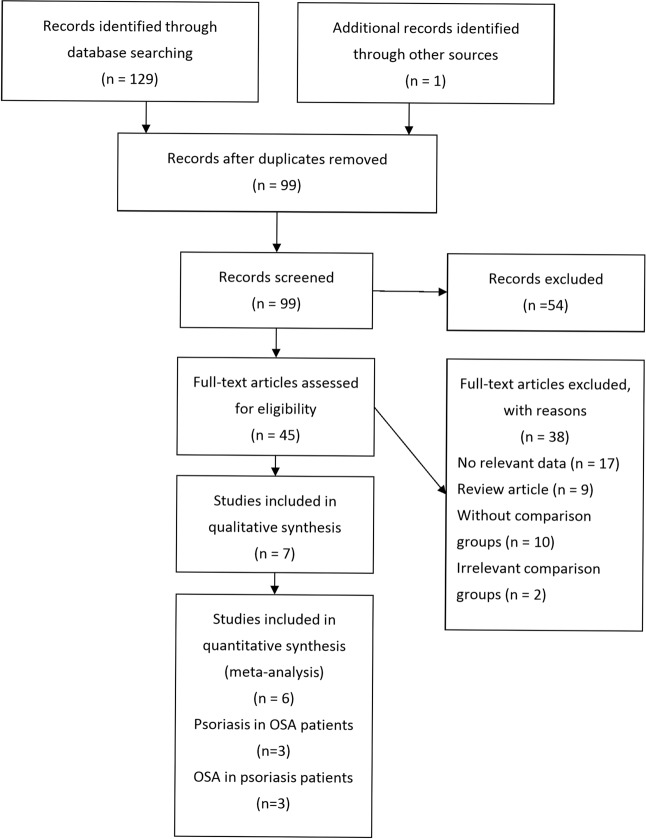
The PRISMA flow chart of study selection is shown in Fig. 1. Our systematic literature search yielded 98 records after removing duplicates. We identified one additional relevant study in a review article. After screening the titles and abstracts, 54 records were excluded. After assessing the full text for eligibility, 38 articles were excluded due to no relevant data, review articles, no comparison group or irrelevant comparison group. Ultimately seven studies with a total of 5,840,495 study subjects were included. One cohort study, two case control studies, and one cross-sectional study investigated the association of psoriasis with OSA. Three cohort studies and one case-control study investigated the association of OSA with psoriasis. There was one cohort study that reported the bidirectional association between psoriasis and OSA. The main characteristics of included studies are summarized respectively in Table 1.
Figure 1.
PRISMA study flow diagram.
Table 1.
Characteristics of included studies.
| First author, year, country | Study design | Case group | Control group | Case definition and sampling population/outcome definition | Results | |
|---|---|---|---|---|---|---|
| Studies investigating the odds of obstructive sleep apnea in psoriasis patients | ||||||
| Tsai, 2011, Taiwan | Case-control | 51,800 patients with psoriasis (31,923 males and 19,877 females) | 207,200 age-, gender- and urbanization- matched controls (127,692 males and 79,508 females) | ICD-9-CM psoriasis code from national health insurance database in 2006/ICD-9-CM sleep apnea code | Crude OR: 3.30 (2.00–5.44) | Adjusted RR: 3.89 (2.26–6.71) |
| Shalom, 2016, Israel | Case-control | 12,336 patients with psoriasis (6,441 males and 5,895 females) | 24,008 age- and sex- matched controls (12,096 males and 11,912 females) | Diagnosis of psoriasis by dermatologists from medical database of Clalit Health Services/ICD-9-CM sleep apnea code | OR:1.74 (1.50–2.03) | Adjusted OR: 1.27 (1.08–1.49) |
| Egeberg, 2016, Denmark | Cohort study | 66,523 patients with psoriasis (32,115 males and 34,408 females) | 5,393,040 individuals in the reference population (2,659,620 males and 2,733,420 females) | ICD-10-CM psoriasis or psoriatic arthritis code from Danish National Patient Register from 1 Jan 1997 to 31 Dec 2011/ICD-10-CM sleep apnea code |
IRR: Mild psoriasis: 1.88 (1.69–2.09) Severe psoriasis: 2.69 (2.00–3.62) Psoriatic arthritis: 3.08 (2.38–4.00) |
Adjusted IRR: Mild psoriasis: 1.36 (1.21–1.53) Severe psoriasis: 1.53 (1.08–2.18) Psoriatic arthritis: 1.98 (1.50–2.61) |
| Sacmaci, 2019, Turkey | Cross-sectional | 60 patients with psoriasis (30 males and 30 females) | 60 sex- and age- matched controls (30 males and 30 females) | Diagnosis of psoriasis by dermatologists//Diagnosis of sleep apnea by neurologists according to Berlin Questionnaire for Sleep Apnea | Crude OR: 6 (1.89–19.04) | NA |
| Studies investigating the odds of psoriasis in obstructive sleep apnea patients | Crude (95% CI) |
Adjusted (95% CI) |
||||
| Yang, 2012, Taiwan | Cohort study | 2,258 patients with sleep apnea (1,414 males and 844 females) | 11,255 age- and sex-matched controls without sleep apnea and psoriasis | ICD-9-CM sleep apnea codes from national health insurance database from 1 Jan 2001 to 31 Dec 2005 after receiving polysomnography/Two consensus psoriasis diagnosis, with at least one made by dermatologist or rheumatologists | Crude HR: 2.21 (1.08–4.49) | Adjusted HR: 2.30(1.13–4.69) |
| Cohen, 2015, USA | Cohort study | 490 patients with OSA (All females) | 71,108 individuals in the reference population (All females) | Self-reported sleep apnea in 1997/Self-reported psoriasis, psoriasis within 2 years of onset of sleep apnea were excluded |
Crude IRR: 2.65 (1.6–4.14) |
Adjusted RR: 1.91(1.20–3.05) |
| Egeberg, 2016, Denmark | Cohort study | 39,908 patients with sleep apnea (31503 males and 8405 females) | 5,419,655 individuals in the reference population (2,660,232 males and 2,759,423 females) | ICD-10-CM sleep apnea code from Danish National Patient Register from 1 Jan 1997 to 31 Dec 2011/ICD-10-CM psoriasis or psoriatic arthritis code |
IRR: Sleep apnea without CPAP Mild psoriasis: 1.97 (1.71–2.26) Severe psoriasis: 2,80 (2.02–3.87) Psoriatic arthritis: 2.38 (1.65–3.34) Sleep apnea with CPAP Mild psoriasis: 2.29 (1.79–2.93) Severe psoriasis: 4.77 (2.96–7.67) Psoriatic arthritis: 7.29 (4.88–10.88) |
Adjusted IRR: Sleep apnea without CPAP Mild psoriasis: 1.62 (1.38–1.89) Severe psoriasis: 1.85 (1.25–2.74) Psoriatic arthritis: 1.98 (1.32–2.99) Sleep apnea with CPAP Mild psoriasis: 1.95 (1.48–2.57) Severe psoriasis: 3.75 (2.22–6.34) Psoriatic arthritis: 6.84 (4.49–10.4) |
| Papadavid, 2017, Greece | Case-control | 253 patients with OSA (200 males and 53 females) | 104 controls without OSA (82 males and 22 females) | Underwent full nocturnal polysomnography, cessation of airflow for ≥10 s, from July 2009 to July 2012/Diagnosed for psoriasis by the same dermatologist | NA | Adjusted OR: 13.31 (1.19–48.93) |
CI, confidence interval; CM, Clinical Modification; HR, hazard ratio; ICD, International Classification of Disease; IRR, incidence rate ratio; N/A, not available; OR, odds ratio; OSA, obstructive sleep apnea; RR, risk ratio.
Risk of bias of included studies
The risk of bias of included studies was summarized in Figs. 2 and 3. Of four included case-control and cross-sectional studies, no items were rated as high risk of bias (see Fig. 2). The Tsai 2011 study was rated with an unclear risk of bias in the ‘adequacy of case definition’ domain because only the International Classification of Disease (ICD) diagnostic codes were used to identify patients33. Two studies were rated with an unclear risk of bias in the ‘representativeness of cases’ and ‘selection of controls’ domains because patients were selected from hospital not community34,35. We rated two studies as unclear risk of bias in the ‘comparability of cases and controls’ domain because only age and gender, but not body mass index, were controlled33,35. Moreover, three studies were rated as unclear risk in the ‘ascertainment of exposure’ domain because only medical records or ICD codes were employed24,33,34. In three included cohort studies, we rated the Cohen 2015 study at high risk of bias in the ‘representativeness of exposed cohort’ domain (see Fig. 3)23. The reason was that the study subjects were all female nurses.
Figure 2.
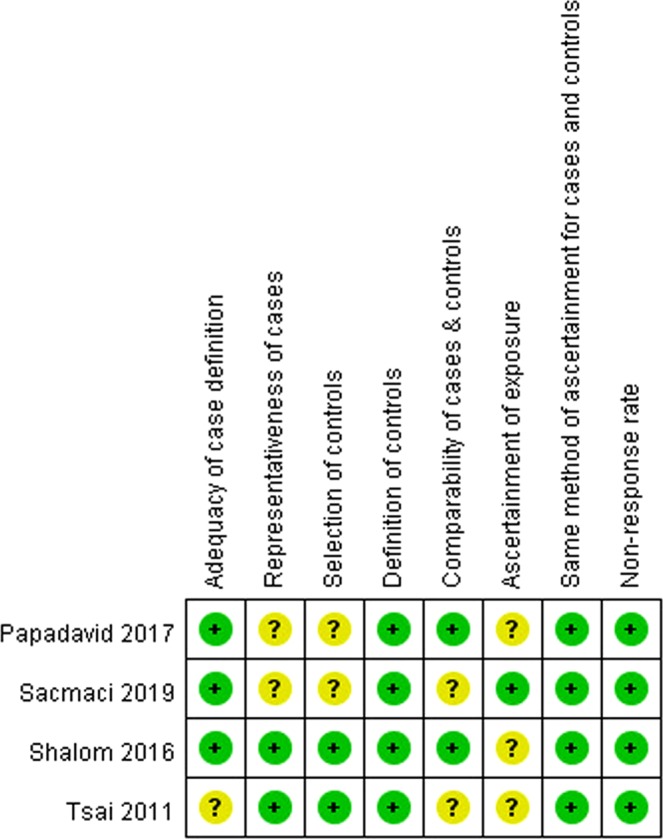
Risk of bias of included case-control and cross-sectional studies. Risk of bias were assessed base on Newcastle-Ottawa Scale. Green dots indicate low risk of bias; yellow dots indicate unclear risk of bias and red dots indicate high risk of bias.
Figure 3.
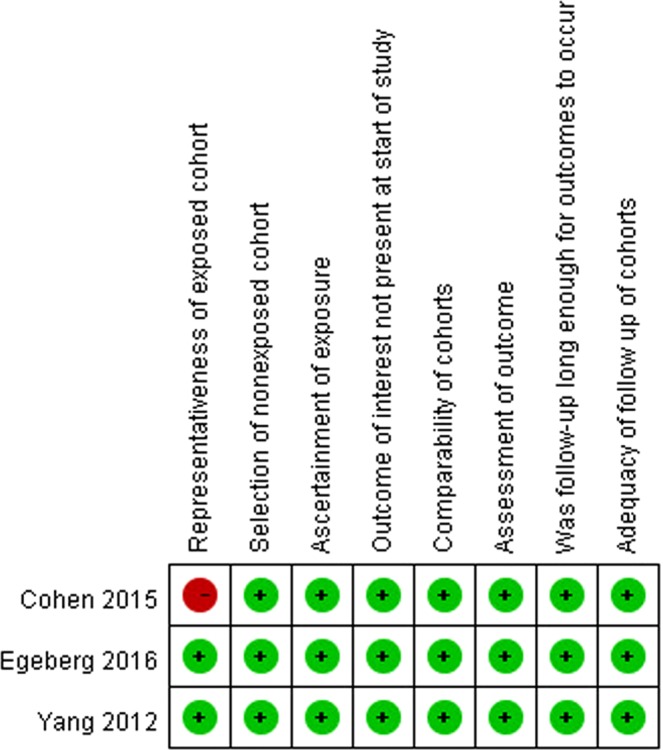
Risk of bias of included cohort studies. Risk of bias were assessed base on Newcastle-Ottawa Scale. Green dots indicate low risk of bias; yellow dots indicate unclear risk of bias and red dots indicate high risk of bias.
Bidirectional association between OSA and psoriasis
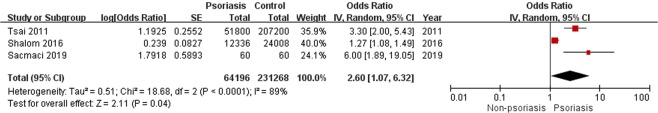
One cohort study25, one cross-sectional study35, and two case-control studies24,33 demonstrated an increase in OSA among patients with psoriasis. The meta-analysis of two included case-control and one cross-sectional studies with 295,464 study subjects showed a significant association of psoriasis with OSA (pooled OR, 2.60; 95% CI, 1.07–6.32; Fig. 4)24,33,35. Considerable statistical heterogeneity was found (I2 = 89%; P < 0.0001). One included cohort study illustrated a consistently increased risk for OSA in patients with mild psoriasis (adjusted IRR 1.36; 95% CI 1.21–1.53), severe psoriasis (adjusted IRR 1.53; 95% CI 1.08–2.18), and psoriatic arthritis (adjusted IRR 1.98; 95% CI 1.50–2.61)25.
Figure 4.
Forest plot for case-control and cross-sectional studies on the association of psoriasis with obstructive sleep apnea. The meta-analysis illustrated a significant association of obstructive sleep apnea with psoriasis (pooled odds ratio 2.60; 95% confidence interval 1.07–6.32).
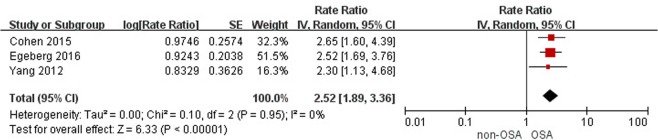
Conversely, three cohort studies23,25,27 and one case-control study34 found a consistent increase in psoriasis among patients with OSA. The meta-analysis of three included cohort studies with 5,544,674 study subjects showed a significant association of OSA with psoriasis (pooled IRR, 2.52; 95% CI, 1.89–3.36; Fig. 5)23,25,27. There was no statistical heterogeneity within these studies (I2 = 0%). The Cohen 2015 study was rated with a high risk of bias in the representativeness of the exposed cohort because all the study subjects were nurses23. However, the association of OSA with psoriasis remained positive after excluding the Cohen 2015 study (pooled IRR 2.47; 95% CI 1.74–3.49). One case-control study showed a significantly increased odds for psoriasis in relation to OSA (adjusted OR 13.31, 95% CI 1.19–48.93)34.
Figure 5.
Forest plot for cohort studies on the association of obstructive sleep apnea with psoriasis. The meta-analysis illustrated a significant association of obstructive sleep apnea with psoriasis (pooled incidence rate ratio 2.52; 95% confidence interval 1.89–3.36).
Discussion
To the best of our knowledge, the present study is the first meta-analysis investigating the bidirectional association between psoriasis and OSA. The evidence from included case-control and cross-sectional studies found a 2.6-fold greater odds for prevalent OSA in relation to psoriasis24,33,35 and a 13-fold increased odds for prevalent psoriasis in relation to OSA34. Similarly, the evidence from an included cohort study demonstrated consistently increased risk for incident OSA among patients with mild and severe psoriasis as well as psoriatic arthritis25. Conversely, OSA patients were 2.52-fold more likely to develop incident psoriasis when compared with non-OSA controls23,25,27. Our meta-analysis possesses high generalizability because of including studies from various ethnicities for example the United States, France, Denmark, Greece, Israel, and Taiwan.
Obesity is a trigger for inflammation and has been linked to chronic inflammatory diseases36. Patients with psoriasis have been found to have increased body mass index (BMI) than healthy controls37. Besides, OSA was related to obesity and thus weight loss is the recommended fist-line therapy38,39. Obesity may promote upper airway inflammation, reduce muscle contractibility, and induce airway collapse during sleep40. BMI has been correlated with psoriasis severity37 and OSA26. Among most of included studies in our meta-analysis23–25,27,34, the confounding from obesity has been considered and BMI was adjusted in the statistical analysis. The bidirectional association between psoriasis and OSA remained significant after adjustment for obesity or BMI. Only one included case-control study of Tsai 2011 did not contain any information about BMI or body weight33. Another included cross-sectional study of Sacmaci 2019 did not perform adjustment for obesity, but there was no significant difference in BMI between psoriasis patients and non-psoriasis controls (28.5 kg/m2 vs. 26.9 kg/m2; P = 0.051)35. Therefore, confounding by obesity could not fully explain the association between psoriasis and OSA.
The bidirectional association between psoriasis and OSA suggest the shared common systemic inflammatory pathogenic pathways19. OSA is considered a systemic inflammatory disorder. High levels of systemic inflammatory molecules are part of mechanism leading to OSA41,42. The activities of IL-17, TNF, IL-6, IL-7 and C-reactive protein are significantly increased in patients with OSA compare to obese patients43–45. These pro-inflammatory cytokines decreased after continuous positive airway pressure (CPAP) treatment44. Studies have shown that the circadian rhythm of TNF release was significantly disturbed in patients with OSA46,47. The inflammatory process may predispose them to the development of psoriasis27. Elevated circulating levels of IL-17, TNF, IL-6, and IL-22 were also associated with psoriasis48. One previous study found that treatment with etanercept significantly improved symptoms of OSA in moderate to severe psoriasis49. The increase of IL-17 is related to atherosclerotic vascular disease, which is a risk factor for OSA50. Moreover, psoriasis causes pruritus and may lead to sleep disturbance, which contributes to systemic inflammation and consequently induces OSA51–53.
Autonomic activation is considered an important factor between OSA and psoriasis53–55. Some scholars assumed that psoriasis-related itching and pain could disrupt sleep and thus increase autonomic activation, which possibly induces OSA18,54. On the other hand, Gabryelska et al. proposed low grade inflammation in OSA patients with elevated levels of IL-1 and TNF could stimulate hypothalamus and increase hypothalamic-pituitary-adrenal activity with resultant surging of autonomic activity56. The inflammation and autonomic activation involved in OSA may be a risk factor for psoriasis55.
Intermittent hypoxia during sleep in OSA patients induces oxidative stress. Some markers including nuclear factor-κB (NF-κB) and hypoxia-inducible factor-1α (HIF-1α) could detect the oxygen stress and activate the inflammatory pathway57. HIF-1α, a transcriptional regulator of cell oxygen metabolism, is a specific marker for diagnosing OSA58. The levels of HIF-1α correlate with the severity of OSA and can be effectively reduced by CPAP treatment57. Besides, vascular risk response to hypoxia was detected in OSA patients. A significant upregulation of NF-κB, HIF-1α, endothelial nitric oxide synthase, vascular cell adhesion molecule 1, and vascular endothelial growth factor (VEGF) were expressed in the skin biopsy specimens of OSA patients59. Increased circulation of VEGF promotes angiogenesis which is also an important process in initiating psoriasis59,60. VEGF is a marker of tissue response to hypoxia and activated by HIF-1α59. There is evidence indicating activation of excess HIF-1α and VEGF in psoriatic skin when compare to normal skin61,62. In addition, HIF-1α is involved in T-cell regulation and survival which are crucial in psoriasis formation62. The effects of hypoxia with elevated levels of HIF-1α and VEGF may explain the association between OSA and psoriasis.
There are a few limitations of the present study. First, OSA was identified by the use of ICD codes in four included studies24,25,27,33 and misclassification bias might have been present. Second, high statistical heterogeneity was detected as to the association of psoriasis with OSA (I2 = 89%; see Fig. 4), but all the include studies consistently reported positive association.
In conclusion, the evidence to date supports a bidirectional association between psoriasis and OSA. All patients with psoriasis should be informed about the risk of OSA, and vice versa. OSA is a comorbidity of psoriasis that should not be ignored, and sleep quality should be inquired in patients with psoriasis. Respirologist consultation or polysomnography may be indicated for those presenting with night snoring, daytime sleepiness, and insomnia.
Supplementary information
Author contributions
This study was designed by C.C. Data extraction was performed by T.G. and Y.F. Data review was done by all authors. First draft was written by T.G. All authors commented on the first draft and agreed with the final version. C.C. is the guarantor of the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62834-x.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017;31:205–212. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–873. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CE, et al. Psoriasis and psoriatic arthritis: immunological aspects and therapeutic guidelines. Clin. Exp. Rheumatol. 2006;24:S72–78. [PubMed] [Google Scholar]
- 5.Machado-Pinto, J., Diniz Mdos, S. & Bavoso, N. C. Psoriasis: new comorbidities. An. Brasileiros de. Dermatologia91, 8–14, 10.1590/abd1806-4841.20164169 (2016). [DOI] [PMC free article] [PubMed]
- 6.Malkic Salihbegovic E, Hadzigrahic N, Cickusic AJ. Psoriasis and metabolic syndrome. Med. Arch. 2015;69:85–87. doi: 10.5455/medarh.2015.69.85-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi CC, et al. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J. Dermatol. Sci. 2015;78:232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Chi, C. C. et al. Risk of uveitis among people with psoriasis: A nationwide cohort study. JAMA Ophthalmol.135, 415–422, 10.1001/jamaophthalmol.2017.0569 (2017). [DOI] [PMC free article] [PubMed]
- 9.Wang SH, Wang J, Lin YS, Tung TH, Chi CC. Increased risk for incident thyroid diseases in people with psoriatic disease: A cohort study. J. Am. Acad. Dermatology. 2019;80:1006–1012. doi: 10.1016/j.jaad.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 10.Yen, H. & Chi, C. C. Association between psoriasis and vitiligo: a systematic review and meta-analysis. Am. J. Clin. Dermatology20, 31–40, 10.1007/s40257-018-0394-1 (2019). [DOI] [PubMed]
- 11.Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417–1423. doi: 10.1001/jamadermatol.2018.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badran M, Ayas N, Laher I. Insights into obstructive sleep apnea research. Sleep. Med. 2014;15:485–495. doi: 10.1016/j.sleep.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kabeloglu Ilbay, V., Tas, B., Altuntas, M., Atakli, H. D. & Soysal, A. Risk of obstructive sleep apnea syndrome in psoriasis patients. Arch. Iran. Med.22, 137–143 (2019). [PubMed]
- 15.Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence-based practice. J. Am. Board. Family Medicine: JABFM. 2007;20:392–398. doi: 10.3122/jabfm.2007.04.060201. [DOI] [PubMed] [Google Scholar]
- 16.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin. Proc. 2011;86:549–554. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagai K. Obstructive sleep apnea, stroke, and cardiovascular diseases. Neurologist. 2010;16:329–339. doi: 10.1097/NRL.0b013e3181f097cb. [DOI] [PubMed] [Google Scholar]
- 18.Hirotsu C, et al. The bidirectional interactions between psoriasis and obstructive sleep apnea. Int. J. Dermatology. 2015;54:1352–1358. doi: 10.1111/ijd.13026. [DOI] [PubMed] [Google Scholar]
- 19.Gupta MA, Simpson FC, Gupta AK. Bidirectional relationship between obstructive sleep apnea (OSA) and psoriasis: Implications for OSA therapies? J. Clin. Sleep. Med. 2016;12:1309. doi: 10.5664/jcsm.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaca S, et al. Might psoriasis be a risk factor for obstructive sleep apnea syndrome? Sleep. Breath. 2013;17:275–280. doi: 10.1007/s11325-012-0686-2. [DOI] [PubMed] [Google Scholar]
- 21.Buslau M, Benotmane K. Cardiovascular complications of psoriasis: does obstructive sleep apnoea play a role? Acta Dermato-Venereologica. 1999;79:234. doi: 10.1080/000155599750011075. [DOI] [PubMed] [Google Scholar]
- 22.Dalamaga M, Papadavid E, Vlami K. Unmasking the Janus face of the association between psoriasis, metabolic syndrome and obstructive sleep apnea. Sleep Breath. 2013;17:449–450. doi: 10.1007/s11325-012-0749-4. [DOI] [PubMed] [Google Scholar]
- 23.Cohen, J. M., Jackson, C. L., Li, T. Y., Wu, S. & Qureshi, A. A. Sleep disordered breathing and the risk of psoriasis among US women. Arch. Dermatol. Res.307, 433–438, 10.1007/s00403-015-1536-4 (2015). [DOI] [PubMed]
- 24.Shalom G, Dreiher J, Cohen A. Psoriasis and obstructive sleep apnea. Int. J. Dermatol. 2016;55:e579–e584. doi: 10.1111/ijd.13367. [DOI] [PubMed] [Google Scholar]
- 25.Egeberg A, et al. Psoriasis and sleep apnea: A Danish nationwide cohort study. J. Clin. Sleep. Med. 2016;12:663–671. doi: 10.5664/jcsm.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papadavid E, et al. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J. Eur. Acad. Dermatol. Venereol. 2013;27:820–826. doi: 10.1111/j.1468-3083.2012.04580.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: A longitudinal population-based study. Sleep Med. 2012;13:285–289. doi: 10.1016/j.sleep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Wells, G., Shea, B., O'Connell, J. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014).
- 30.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin. trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Tsai TF, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Dermatol. Sci. 2011;63:40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Papadavid E, et al. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: a large hospital-based case-control study. Sleep. Breath. 2017;21:949–958. doi: 10.1007/s11325-017-1507-4. [DOI] [PubMed] [Google Scholar]
- 35.Saçmacı, H. & Gürel, G. Sleep disorders in patients with psoriasis: a cross-sectional study using non-polysomnographical methods. Sleep Breath., 10.1007/s11325-019-01820-8 (2019). [DOI] [PubMed]
- 36.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmunity Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Duarte GV, et al. Association between obesity measured by different parameters and severity of psoriasis. Int. J. Dermatol. 2013;52:177–181. doi: 10.1111/j.1365-4632.2011.05270.x. [DOI] [PubMed] [Google Scholar]
- 38.Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep. 1996;19:104–115. doi: 10.1093/sleep/19.2.104. [DOI] [PubMed] [Google Scholar]
- 39.Foster GD, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch. Intern. Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch. Physiol. Biochem. 2008;114:211–223. doi: 10.1080/13813450802364627. [DOI] [PubMed] [Google Scholar]
- 41.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am. J. Respir. Crit. Care Med. 1996;153:1080–1086. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- 42.Carpagnano GE, et al. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 43.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/circulationaha.105.556746. [DOI] [PubMed] [Google Scholar]
- 44.Minoguchi K, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 45.Yue HJ, et al. The roles of TNF-alpha and the soluble TNF receptor I on sleep architecture in OSA. Sleep Breath. 2009;13:263–269. doi: 10.1007/s11325-008-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strober, B. E. et al. Sleep quality and other patient-reported outcomes improve after patients with psoriasis with suboptimal response to other systemic therapies are switched to adalimumab: results from PROGRESS, an open-label Phase IIIB trial. Br. J. Dermatol. 167, 1374–1381, 10.1111/bjd.12000 (2012). [DOI] [PubMed]
- 47.Sacmaci H, Gurel G. Sleep disorders in patients with psoriasis: a cross-sectional study using non-polysomnographical methods. Sleep Breath. 2019 doi: 10.1007/s11325-019-01820-8. [DOI] [PubMed] [Google Scholar]
- 48.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273–279. doi: 10.1155/mi.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaçi, D. et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J. Eur. Acad. Dermatol. Venereol.28, 900–906, 10.1111/jdv.12207 (2014). [DOI] [PubMed]
- 50.Hashmi S, Zeng QT. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 2006;17:699–706. doi: 10.1097/01.mca.0000236288.94553.b4. [DOI] [PubMed] [Google Scholar]
- 51.Shutty, B. G. et al. Sleep disturbances in psoriasis. Dermatology Online J.19, 1 (2013). [PubMed]
- 52.Yosipovitch, G., Goon, A., Wee, J., Chan, Y. H. & Goh, C. L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 143, 969–973, 10.1046/j.1365-2133.2000.03829.x (2000). [DOI] [PubMed]
- 53.Gabryelska A, Sochal M, Wasik B, Bialasiewicz P. Patients with obstructive sleep apnea are over four times more likely to suffer from psoriasis than the general population. J. Clin. Sleep. Med. 2018;14:153. doi: 10.5664/jcsm.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta MA, Gupta AK. Psoriasis is associated with a higher prevalence of obstructive sleep apnea and restless legs syndrome: A possible indication of autonomic activation in psoriasis. J. Clin. Sleep. Med. 2018;14:1085. doi: 10.5664/jcsm.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabryelska A, Bialasiewicz P. Is autonomic activation a middleman between obstructive sleep apnea syndrome and psoriasis? J. Clin. Sleep. Med. 2018;14:1087–1088. doi: 10.5664/jcsm.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spath-Schwalbe, E., Gofferje, M., Kern, W., Born, J. & Fehm, H. L. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol. Psychiatr. 29, 575–584, 10.1016/0006-3223(91)90093-2 (1991). [DOI] [PubMed]
- 57.Lu D, Li N, Yao X, Zhou L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn. J. basic. Med. Sci. 2017;17:47–53. doi: 10.17305/bjbms.2016.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabryelska, A. et al. Serum Hypoxia-Inducible Factor-1alpha protein level as a diagnostic marker of obstructive sleep apnea. Pol. Arch. Intern. Med., 10.20452/pamw.15104 (2019). [DOI] [PubMed]
- 59.Kaczmarek E, et al. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS one. 2013;8:e70559. doi: 10.1371/journal.pone.0070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marina ME, et al. VEGF involvement in psoriasis. Clujul Med. 2015;88:247–252. doi: 10.15386/cjmed-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Detmar, M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J. Investig. Dermatol. 122, xiv–xv, 10.1046/j.0022-202X.2003.22140.x (2004). [DOI] [PubMed]
- 62.Rosenberger, C. et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin. J. Invest. Dermatol. 127, 2445–2452, 10.1038/sj.jid.5700874 (2007). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.