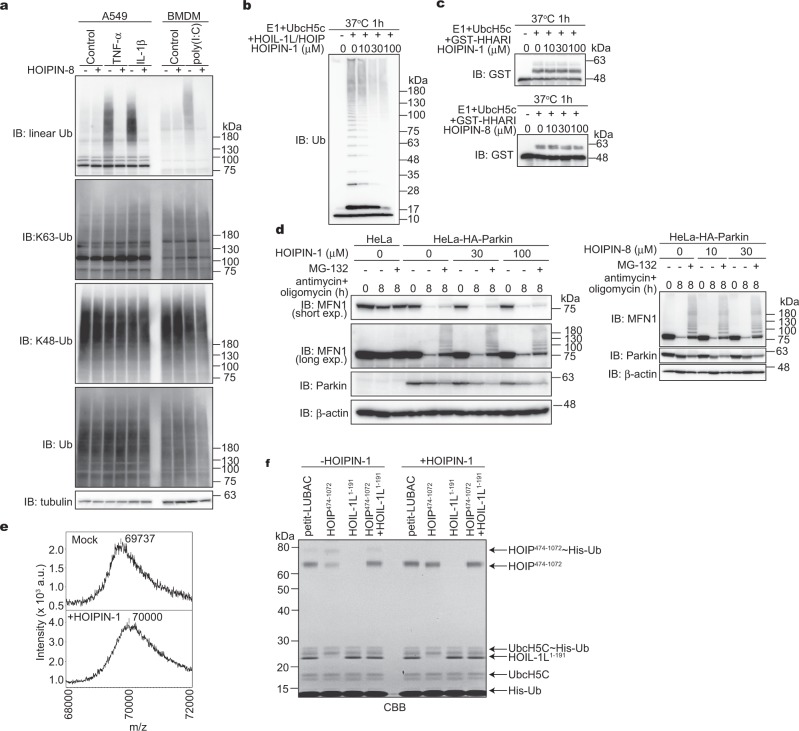
Fig. 3. HOIPINs suppress the RING-HECT-hybrid reaction of LUBAC.
a HOIPIN-8 selectively suppresses the linear ubiquitin level. A549 cells and BMDM were pre-treated with 30 μM HOIPIN-8, and either 10 ng/ml TNF-α, 1 ng/ml IL-1β, or 10 μg/ml poly(I:C) for 1 h with HOIPIN-8, and cell lysates were immunoblotted with the depicted antibodies. b, c Effects of HOIPINs on RBR-type E3 activities. In vitro ubiquitination assays for baculovirus-expressed recombinant LUBAC, composed of full-length HOIL-1L and HOIP (b), and auto-ubiquitination activities of GST-HHARI (c) were examined in the presence of the indicated concentrations of HOIPIN-1 or HOIPIN-8, and immunoblotted with the indicated antibodies. d HOIPIN-8 does not suppress the parkin-dependent ubiquitination of mitofusin-1 (MFN1). Parental HeLa and stable HA-parkin-expressing HeLa cells were pre-treated with the indicated concentrations of HOIPINs, and then treated with 5 μM antimycin and 10 μM oligomycin for the indicated times, in the presence of HOIPINs, with or without 10 μM MG-132. The cell lysates were then immunoblotted with the indicated antibodies. e HOIPIN-1 stoichiometrically binds to the C-terminal portion of HOIP. Petit-LUBAC was treated with 100 μM HOIPIN-1 for 1 h, and the molecular mass of HOIPIN-1-treated petit-LUBAC was analyzed by mass spectrometry. f HOIPIN-1 inhibits the RING-HECT-hybrid reaction in HOIP. In vitro ubiquitination and thioester-linked ubiquitin-binding assay was performed using E1, UbcH5C, His-ubiquitin, and LUBAC components in the presence or absence of 30 µM HOIPIN-1 as indicated. Samples were electrophoresed under non-reducing conditions and stained with Coomassie Brilliant Blue (CBB).