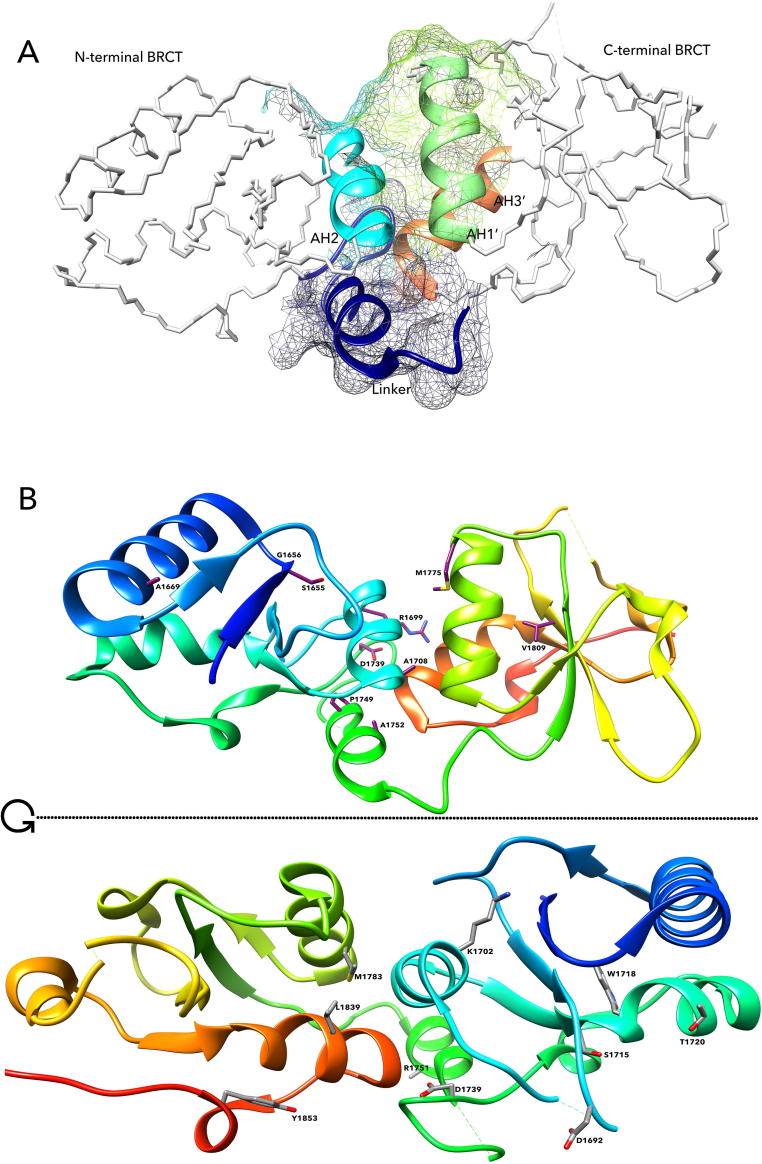
Fig. 1.
Structure of BRCT repeats. (A). Features of the structure. Each BRCA1 BRCT repeat shown as alpha carbon trace is comprised of a central, four stranded β sheets, surrounded by two α helices (α1 and α3) on one face and a single helix (α2) on the opposite face of β sheet. The AH2, AH3′ and AH1′ along with the linker helix forming the phosphor-peptide binding motif are shown in ribbon conformation. (B). Amino acid positions within BRCT phosphor-peptide binding motif in the hydrophobic cleft involved in phospho-peptide binding. The up part and the low part are 180-degree horizontal turn to show the variants on both sides of BRCA1 BRCT repeats. The up part shows the variant S1655, D1692, K1702, F1704, A1708, S1715, P1749, M1775, L1839, Y1853, the low part shows the variant D1692, K1702, S1715, W1718, T1720, D1739, R1751, M1783, L1839, and Y1853.