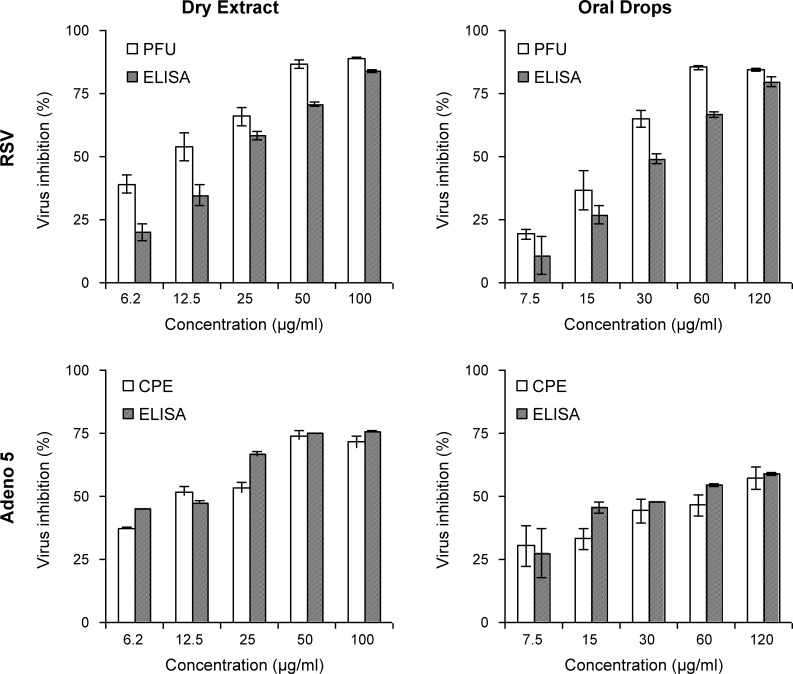
Fig. 2.
Ascertainment of antiviral activity of two Sinupret® preparations with different assays. To test the efficacy of the two Sinupret® preparations – oral drops and dry extract – HEp-2 cells were infected with RSV (M.O.I. of 0.0004) or with Adeno 5 (M.O.I. of 0.008). After infection, cell monolayers were incubated without (medium-control) or in the presence of five descending non-cytotoxic concentrations (x-axis, μg/ml) of the test substances oral drops and dry extract. The antiviral activity of Sinupret® against the viruses was determined with plaque reduction assays in plaque forming units (PFU) for RSV or by analyses of a cytopathogenic effect (CPE) for Adeno 5 (open columns) and with the quantification of the amount of newly synthesised virus by enzyme immunoassays (ELISA; striped columns). The relative inhibition (y-axis, % inhibition) was calculated by analysing the number of plaques, the lesions of the viral CPE or the amount of the viral proteins of the respective groups and standardised by the virus control representing 100% infectivity (0% inhibition). The results of the antiviral activity against RSV are shown in the upper, against Adeno 5 in the lower panels. Positive controls confirmed the procedure (Adeno 5, laboratory standard 7.5 μg/ml, CPE 57% and ELISA 53% inhibition; RSV 6 μg/ml Ribavirin, PFU 60% and ELISA 57% inhibition). All data represent means and SEM from two independent experiments with two (PFU and CPE) to three (ELISA) replicates.