Figure 1.
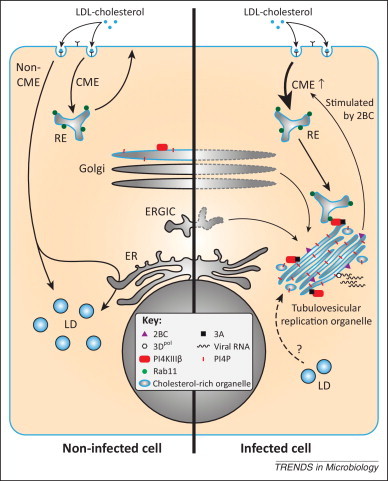
Enteroviruses alter the cellular cholesterol landscape to support virus replication. In uninfected cells (left side), extracellular cholesterol from low-density lipoprotein (LDL) particles and free cholesterol from the plasma membrane are taken up via clathrin-mediated endocytosis (CME) and distributed to cellular membranes, e.g., via recycling endosomes (RE) to the plasma membrane. Cholesterol that enters via non-CME is stored in lipid droplets (LD) in a caveolin-dependent manner. In enterovirus-infected cells (right side), membranes from the endoplasmic reticulum (ER)–Golgi intermediate compartment (ERGIC) and the Golgi apparatus are remodeled into tubulovesicular replication organelles (ROs) that are rich in cholesterol. At these ROs, the viral RNA-dependent RNA polymerase, 3Dpol, replicates the viral RNA. The viral protein 2BC enhances CME and cholesterol uptake (the fate of non-CME in infected cells is unknown and therefore not depicted). Following its normal route, endocytosed cholesterol is delivered into REs. Viral protein 3A mediates the recruitment of phosphatidylinositol-4-kinase IIIβ (PI4KIIIβ), which produces large amounts of phosphatidyl-4-phosphate (PI4P) lipids at the ROs. PI4KIIIβ in turn attracts cholesterol-rich REs through a direct interaction with the RE protein Rab11, resulting in the delivery of cholesterol to ROs. Consequently, storage of excess cholesterol in LDs is inhibited during enterovirus infection. It is remains to be established whether cholesterol stored in LDs is mobilized and shuttled to ROs as an additional source.
