Abstract
The trachea is a pivotal organ of the respiratory tract. Rather than a genuine anatomic border, it acts as a crossroad in all respiratory infectious processes. Even though not strictly limited to the trachea, infections such as laryngotracheitis and tracheobronchitis are frequently diagnosed in children, in particular during the winter season. Infectious tracheitis etiologies are diverse and the distinction between viral and bacterial origins, albeit difficult, remains relevant considering the substantial differences in terms of gravity and therapeutic management. This literature review summarizes the microbiological and clinical aspects of community-acquired and nosocomial tracheitis in adults and children, as well as the adequate diagnostic and therapeutic approaches. It also highlights the emergence of fungal tracheitis in immunocompromised patients, of ventilator-associated tracheitis in intensive care medicine, and beyond all that the potential short and long-term consequences of tracheitis.
Keywords: Tracheitis, Croup, Ventilator-associated tracheitis
Résumé
Considérée comme le plus long organe des voies respiratoires, la trachée joue plus un rôle de carrefour au cours des processus infectieux, qu’une véritable frontière anatomique. Alors que la trachéite pure est rare, les laryngo-trachéites et les trachéo-bronchites sont fréquemment diagnostiquées chez l’enfant en période hivernale. Le spectre étiologique des trachéites infectieuses est vaste et la distinction entre origine virale et bactérienne reste un véritable challenge tant la gravité et la gestion thérapeutique varie entre ces deux origines. Cette revue souligne les particularités microbiologiques et cliniques de l’enfant et de l’adulte au cours des trachéites communautaires et nosocomiales, et décrit les approches diagnostique et thérapeutique. Elle met en exergue l’émergence des trachéites fongiques chez le patient immunodéprimé comme la trachéo-bronchite aspergillaire, et les trachéites acquises sous ventilation chez le patient en réanimation, et au-delà de ces situations spécifiques, les conséquences à court et long terme des trachéites.
Mots clés: Trachéite, Croup, Trachéite acquise sous ventilation
1. Introduction
Infectious tracheitis is always neglected because of the concomitant and frequent involvement of adjacent airways, the associated non-specific clinical signs, and the endoscopy required to confirm the diagnosis.
However, several factors urge a particular attention to the tracheal infection. Distinguishing a viral infection from a bacterial one in pediatric patients, and a colonization from a tracheal infection in patients admitted to the Intensive Care Unit (ICU) is a real challenge. Short-term consequences of tracheitis may be fatal and must be well-known. The same goes for longer term consequences of post-infectious stenosis. Infectious tracheitis should be viewed as an entity in its own right considering the specificities of its management.
2. The trachea: an organ at the crossroad of infectious processes
The trachea is the longest part of the airways. It prolongs the larynx and separates to form the main bronchial tubes. The trachea is made of fibers, muscles, and cartilages and can thus resist to the various pressures applied by the breathing mechanism and depress itself during the inspiratory collapse. Its mucous membrane is made of a pseudo-stratified ciliated respiratory epithelium with goblet cells that are responsible for mucociliary activity and for drainage of glandular secretions towards the pharynx. The presence of bronchial-associated lymphoid tissue (BALT) ensures cellular or humoral mucosal immunity [1].
Tracheitis is an inflammation of the trachea, which may be infectious. “Isolated” tracheitis is rare. The inflammation usually affects surrounding organs such as the larynx (laryngotracheitis) and/or the bronchial tubes (tracheobronchitis, laryngotracheobronchitis [LTB]), depending on the infectious agent involved and on the pathophysiology of the infection (direct, toxinic, etc.) (Table 1 ). Acute LTB or “croup” is considered when signs and symptoms of viral laryngotracheitis or spasmodic croup of viral and/or atopic origin are observed in children aged below 6 years [2]. Inflammation of the tracheal and/or laryngeal wall(s) leads to a narrower lumen and to an increased airflow in this area (Venturi effect). This constriction triggers a negative pressure responsible for the airway collapse, which in turn triggers turbulent airflow, known as stridor. Stridor can be inspiratory when the obstruction originates in the larynx or cervical trachea, or expiratory when the obstruction originates in the intrathoracic trachea or in the bronchial tubes. It can even be biphasic when the inflammatory obstruction is diffuse [3].
Table 1.
Etiologies of infectious tracheitis.
Spectre étiologique des trachéites infectieuses en fonction des situations.
| Context | Children | Adults |
|---|---|---|
| Community-acquired | ||
| Viral | ||
| Acute community-acquired presentations | Viruses: parainfluenza 1 and 2, influenza A and B, RSV, adenovirus, rhinovirus, coronavirus, echovirus, enterovirus, coxsackievirus, morbillivirus, paramyxovirus | |
| HSVa (reactivations) VZV (primary infection) |
||
| Bacterial | ||
| Acute community-acquired presentations |
Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae Group A Streptococcus, Moraxella catarrhalis, Mycoplasma pneumoniae |
Staphylococcus aureus, Haemophilus influenzae Group A Streptococcus |
| Progressive outcome | Mycobacterium tuberculosisa, Bordetella pertussis | |
| Unvaccinated, migrant | Corynebacterium diphtheriae | |
| Rare | Corynebacterium pseudodiphtheriticum, Actinomyces, Treponema pallidum (gumma), Klebsiella rhinoscleromatis (scleroma) | |
| Fungal | ||
| Frequent | Aspergillusa (immunodeficiency, burn patients, mechanical ventilation) | |
| Diabetes, influenza | Zygomycetesa | |
| HIV, AIDS stage | Cryptococcus neoformansa | |
| Coming from specific regions of the world | Coccidioidesa | Histoplasmaa, Penicillium, Coccidioidesa, Paracoccidioidomycesa, Blastomyces |
| Nosocomial/healthcare-associated: (tracheostomy and VABT) | ||
| Viral | ||
| Mechanical ventilation | HSV (reactivations) | |
| Bacterial | ||
| Mechanical ventilation, tracheostomy (often multimicrobial) | Gram-negative bacilli (in order of frequency): Pseudomonas aeruginosa, Haemophilus influenzae, Klebsiella, Escherichia coli, Enterobacter cloacae. Gram-positive cocci: Staphylococcus aureus, Streptococcus pneumoniae | Gram-negative bacilli, 75% (in order of frequency): Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella, Escherichia coli, Enterobacter Gram-positive cocci: Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus |
| Immunodeficiency | Bacillus cereus | |
| Fungal | ||
| Cellular immunodeficiency |
Aspergillus Zygomycetesa, Fusarium, Cryptococcus neoformans |
|
RSV: respiratory syncytial virus; HSV: herpes simplex virus; AIDS: acquired immune deficiency syndrome; VABT: ventilator-associated bacterial tracheobronchitis; VZV: varicella zoster virus.
Isolated tracheal presentations described.
Anatomical differences between children and adults make children at higher risk of severe signs and symptoms during upper respiratory tract infection. The subglottic region is the narrower portion of the airways in children, while it is the glottis in adults. This subglottic region is surrounded by cricoid cartilage, which is itself covered by a loose mucous membrane prone to edema during the infection.
Children presenting with tracheitis often have symptoms of airway obstruction that may include respiratory distress syndrome. These symptoms include tachypnea, stridor, and hoarse voice. Patients usually present with an initially dry, hoarse, painful, and spasmodic cough (coughing fit) that may evolve into a productive cough. Cough usually occurs at night, and its deterioration may be due to the lying position or to the circadian rhythm of cortisol and epinephrine levels with low levels between 11pm and 4am, just like asthma peaks that are more often observed at night [4]. Stridor is indicative of the narrowing of the upper airways, that most often originates in the larynx and sometimes in the trachea. Inspiratory and/or expiratory dyspnea, symptoms of respiratory distress syndrome with labored breathing, cyanosis, consciousness disorders, and agitation may occur depending on the extent of the tracheal obstruction. Fever is not always observed in patients presenting with viral tracheitis; it is however more frequent and severe with a bacterial origin. Severity is determined depending on the degree of stenosis (marked/persisting at rest/persisting despite ongoing treatment stridor, sub-clavicle labored respiration) and on the consequences of the lack of oxygen (cyanosis, desaturation, agitation, consciousness disorders) [4]. Pediatric scores may be used to assess croup severity [4].
The main differential diagnoses are epiglottitis (high fever, toxic shock syndrome, absence of hoarse cough, dysphonia, refusal to lie down), tracheal obstacle, angioneurotic edema (sudden without infectious signs), retropharyngeal abscess, laryngeal diphtheria, or allergic reaction.
Signs of airway obstruction in adults are usually less severe. A biphasic stridor must lead physicians to suspect tracheitis, although not always present. Patients most frequently present with a cough, that may sometimes be productive, and dyspnea that may be as severe as respiratory distress syndrome. Unlike children in whom toxic signs and severe respiratory distress syndromes are observed during bacterial LTB, these symptoms are rarely observed in adults [5].
Frontal and lateral (left) chest and cervical region x-ray is the first-line examination for patients presenting with a suspicion of tracheal infection. X-ray results of patients presenting with croup usually reveal an excessive distension of the lower pharyngeal region and a narrowing of subglottic airways; steeple sign may even be observed. For bacterial tracheitis – mainly suspected in patients presenting with toxic clinical signs – the x-ray may reveal a narrowing of subglottic airways and even the presence of intraluminal tracheal membranes or irregularities of the tracheal wall. These signs support the bacterial origin of the infection [6]. The x-ray also reveals pulmonary infiltrates during tracheobronchopneumonia. CT scan is, however, the reference examination to detect the presence of stenosis, of a thickening of the tracheal wall, and of a tracheal obstruction by pseudomembranes. Its main limitation is radiation exposure, especially for young children. Radiation exposure may, however, be reduced with low-dose CT imaging [7]. CT scans are more sensitive and more specific than x-rays for detecting an associated pulmonary infection. Flexible endoscopes help in visualizing supraglottic structures. Subglottic mucosal edema and purulent secretions are often observed with a bacterial infection [8]. Flexible endoscopes help in performing tracheal aspirations to unblock airways and ensure non-contaminated samples for microbiological documentation. The diagnosis may sometimes be confirmed during the autopsy for patients who presented with severe manifestations, most often of bacterial origin [9].
3. Epidemiology and etiologies of infectious tracheitis
3.1. Pediatric community-acquired tracheitis
Viral tracheitis is probably not the adequate term as the larynx, trachea, and bronchial tubes are affected during viral infection. This laryngotracheobronchitis (LTB), or croup [3], mainly affects children from 6 months to 3 years of age, with an incidence peak at the age of 2. LTB may also occur in infants aged below 3 months or in adolescents [2]. The subglottic region is indeed narrower in children than in adults, and is therefore more rapidly obstructed during inflammation. Croup is one of the most frequent causes of respiratory distress in children. Many viruses with a tropism for the lungs may be responsible for tracheal infection in children: influenza A, B, and C viruses, human parainfluenza viruses, metapneumovirus virus, respiratory syncytial virus (RSV), coronavirus, adenovirus, and rhinovirus. These viruses are mainly observed during seasonal epidemics. The authors of a Northern American study observed an incidence peak in autumn, at the same time as that of parainfluenza virus infections, although croup was also reported during summer [10]. Other viruses may be associated with tracheal infection, even though rarely: herpes simplex virus (HSV), varicella zoster virus (VZV), cytomegalovirus (CMV), enterovirus, echovirus, coxsackievirus, or the measles virus (18.6% of LTBs in a study of 440 children presenting with measles [11]).
Pediatric bacterial LTB seems to be less frequently reported, with approximately 0.1/100,000 children-years in the United Kingdom, based on the little available data [12]. Only 400 case patients have been reported in the literature. Median age of onset is 5 years (1–186 months) [12]. Haemophilus influenzae type B and Staphylococcus aureus have long been the main bacteria implicated, followed by streptococci and anaerobes [8]. The H. influenzae type B vaccine was first introduced in the 1980s. Since then, S. aureus accounts for 43.8% of documented cases, followed by H. influenzae (16.5%), Streptococcus pneumoniae (15.3%), Moraxella catarrhalis (10.8%), Streptococcus pyogenes (8.5%), Escherichia coli, and Pseudomonas aeruginosa [12]. Only one multicenter study has been published and, when looked for, viral coinfection was observed in 31% of cases. A clear peak in incidence is observed in winter. Influenza and parainfluenza viruses are most often incriminated [12].
Viral LTB onset is sudden, usually at night, and patients present with the typical barking cough, stridor, and respiratory distress. They also usually present with upper airway non-specific prodromes 12 to 48 hours before viral LTB onset (coryza, pharyngeal pain) [4]. Fever is not always observed [12]. Symptom duration is short; symptoms usually resolve within less than 48 hours in 60% of cases, but may last more than a week in some children. A bacterial origin must be suspected when these symptoms are complicated by worsening of stridor, respiratory distress onset, toxic appearance, high fever, orthopnea, and/or dysphagia [8]. Eckel et al. suggested defining bacterial tracheitis as follows: (1) stridor, (2) respiratory distress, (3) poor or no response to the corticosteroid therapy or to nebulized epinephrine, and (4) presence of purulent secretions, tracheal inflammation, and subglottic narrowing with a normal epiglottis at laryngoscopy or bronchoscopy [13].
Croup diagnosis is based on clinical symptoms. The initial course of the disease (the first few hours) determines the examinations to perform. In the absence of severe signs and symptoms and in case of a favorable outcome, biological samples are not required; it would worsen the patient's respiratory status and stress the child. Otherwise, biological samples help confirm the bacterial origin of the infection when leukocytosis with neutrophils, leukopenia or immature presentations, or an inflammatory syndrome are observed [9]. Blood cultures must be performed when toxic presentations or severe symptoms are observed. They, however, rarely help in establishing the diagnosis [8], [14].
X-rays are not performed in patients presenting with croup when a rapid response to treatment is obtained. They are contraindicated when an epiglottitis is suspected. Frontal and lateral chest and cervical region x-rays are performed when clinical symptoms worsen or in patients presenting with initially severe symptoms. Without distinguishing the viral from the bacterial origin of the infection, cervical region x-rays can usually reveal a subglottic narrowing even though often normal [4], [12]. X-rays help in confirming the bacterial tracheitis diagnosis by visualizing intratracheal opacities corresponding to pseudomembranes or irregularities of the wall. These abnormalities are observed in 9–82% of bacterial tracheitis patients [12], [14]. X-rays also help in looking for pulmonary infiltrates, which are frequently observed in severe presentations [14], [15]. They also help in detecting complications (pneumothorax, pneumomediastinum, lobar atelectasis) [15].
Fibroscopy rules out epiglottitis and confirms the bacterial tracheitis diagnosis by visualizing the subglottic narrowing, a diffuse erythema and a mucopurulent exudate, pseudomembranes that may be responsible for obstructions, ulcerations, or microabscesses on the tracheal mucous membrane. The examination that should be performed is decided on a case-by-case basis according to the child's age, symptom severity, and whether or not a trained operator is available [15]. By allowing a cleaning up of the tracheobronchial tract, secretions are aspirated for microbiological analysis purposes, which leads to the identification of a pathogen in 62–87% of cases [8], [14].
CT scans are much more efficient than x-rays in detecting tracheobronchial abnormalities; they, however, do not impact the management of acute LTB when no signs of severity are observed. The benefits of the CT scan have not been assessed in patients presenting with severe croup, but its main limitation is the resulting radiation and an endoscopy is still required when treatment is proposed. Except for acute presentations, CT scans may be performed in case of recurrent tracheitis episodes to look for tracheal stenosis. This low-dose CT scan has an 87% and 86% sensitivity and specificity, respectively, as compared with bronchial fibroscopy to look for tracheal stenosis [16].
Viral LTB management first requires a calm and comfortable environment to be set up as agitation may worsen respiratory symptoms. Although its benefit has never been proven, oxygen therapy is usually administered in case of respiratory distress if it does not lead to the child's agitation. The authors of a meta-analysis did not observe any benefit to air humidification. Helium administration is also not recommended. However, the benefit of a single dose of corticosteroids has been proven [4]. Its benefit on the hospital length of stay, the risk of readmission for treatment and intubation, and on the duration of mechanical ventilation has indeed been proven. Administering a single dose of corticosteroids does not seem to be less effective than a treatment administered for several days. Dexamethasone should be preferred to prednisone as it is better tolerated and possibly more effective, at doses of 0.15 to 0.60 mg/kg. Oral and intramuscular routes are associated with the same efficacy; inhalation is, however, less effective. The administration of nebulized epinephrine for severe presentations of croup reduces the risk of intubation [4]. The effect of epinephrine administration is temporary (at least 1 hour). Nebulized administration may thus be repeated until clinical improvement, but a reevaluation must be performed two hours after administration. When no improvement is observed or if symptoms deteriorate, patients must be admitted to the ICU and bacterial tracheitis must be suspected. Tracheal intubation is sometimes required [4]. Indeed, 75% of bacterial LTBs reported in the literature required endotracheal intubation. This figure highlights the severity of this disease [12].
Bacterial LTB may be complicated by hypotension (5.5%), even by septic and/or toxinic shock, acute respiratory distress syndrome (2.8%), cardiorespiratory arrest (2.3%), pneumothorax (1.4%) or atelectasis, subglottic stenosis (0.9%), pulmonary edema, or cardiac decompensation [9], [12], [14]. The case fatality rate reached 10–40% during the first half of the 20th century [17] and progressively decreased afterwards with 4% reported in a study of 154 case patients from 1979 to 1998 [14], to finally drop to 1% since the years 2000 [12].
The combination of amoxicillin-clavulanic acid or 3rd-generation cephalosporin are the best choices for patients presenting with LTB. These treatments should then be tailored to the microbiological results if a tracheal aspiration is performed [12]. Treatment duration has not been clearly defined, but the need for more than 7 days of treatment has never been proven.
3.2. Adult community-acquired tracheitis
Adult croup is less frequently observed than pediatric croup; it is a full-fledged illness that refers to upper airway obstruction presentations of community-acquired infectious origin. The above-mentioned seasonal respiratory viruses are responsible for LTB in adults. Tracheobronchitis was, for instance, observed in 20% of symptomatic infections caused by SRV in adults presenting with no comorbidities [18], and in 25% of severe presentations of H1N1 influenza [19].
The authors of another study observed a tracheobronchial involvement in 85% of patients presenting with fatal H1N1 influenza, that was sometimes necrotic and/or hemorrhagic [20]. Death was most often caused by the associated pulmonary infection. The influenza virus tropism for the airways is related to the distribution gradient of some receptors (sialic acids), at the surface of the respiratory mucous membrane. This partly explains why some patients only present with nasopharyngeal manifestations, while others have tracheobronchial presentations or even viral pneumonia. The presence of type 2–6 sialic acids within the trachea probably influences the tracheal infection [21]. Histopathological studies performed during the 1918 pandemic highlighted that a multifocal destruction and pseudo-stratified epithelium desquamation of the trachea and bronchial tubes occur at the early stage. Only the basal layer of the epithelium remains intact and the congestive edema of the submucous membrane is often marked. At more advanced stages, the epithelium regenerate with the formation of a metaplasia by a stratified non-keratinized epithelium [22]. This tracheobronchial involvement results in a local bacterial superinfection followed by a pulmonary infection. This leads to bacterial croup, still called pseudomembranous croup, purulent tracheobronchitis, or membranous LTB [4]. Mucopurulent exudates developed and may cause life-threatening acute obstruction of the airways [8].
Just like in children, bacterial LTB onset follows prodromes (coryza, pharyngeal pain), but unlike children, clinical presentations are usually less severe and the prognosis is far better [5]. To our knowledge, inflammatory biomarkers (C-reactive protein and procalcitonin) have not been investigated to distinguish a viral origin from a bacterial one during tracheitis. A cytobacteriological examination must be performed in case of productive expectorations because of its non-invasive nature. Results must be interpreted based on conventional quality criteria. Its performances have not been assessed during bacterial tracheitis. Imaging tests (first line: x-ray, second line: CT scan) must be performed for severe presentations, tracheal obstruction suspicion, and/or in the absence of improvement despite conventional treatments. Fibroscopy, which should not be performed in patients presenting with viral croup, must however be performed when a bacterial tracheitis is suspected in the emergency department. The benefits of fibroscopy is to clear the airways through extraction of obstructive pseudomembranes. The diagnosis can thus be established by confirming the presence of diffuse deposits of pseudomembranes, of an exudate responsible for the obstruction, and of erosions [23]. Fibroscopy is also useful for microbiological documentation and for collecting potential histological samples [23].
The antibiotic therapy must be active against the main causative agents (Table 1). The combination of amoxicillin-clavulanic acid or an injectable third-generation cephalosporin seem to be the best choices for community-acquired bacterial tracheitis. Pristinamycin is a potential alternative in case of β-lactam allergy. Unlike in children, the corticosteroid therapy is not systematic as the infection is less severe in adults. A corticosteroid therapy is prescribed to patients presenting with non-self-limiting obstruction (dyspnea, persistent stridor) or right away in case of a poor respiratory function.
Presentations may be complicated by tracheal stenosis. Only three cases have been reported following S. aureus tracheitis [24]. A tracheostomy must be rapidly performed when the obstacle cannot be removed and leads to respiratory failure. Expectorations can thus be cleared.
3.3. Nosocomial or healthcare-associated tracheitis
One may distinguish two main presentations:
-
•
bacterial tracheitis in patients with a tracheostomy. A monobacterial or multibacterial colonization is often observed in these patients, i.e. 95% and 83% of cases, respectively. This colonization usually paves the way to the tracheal infection [23]. S. aureus is most often isolated, followed by Gram-negative bacilli, P. aeruginosa, and S. pneumoniae [23];
-
•
ventilator-associated bacterial tracheitis or tracheobronchitis, during which the presence of a tracheal tube reduces the coughing capacity, leads to tracheal ulcerations and fosters a local multibacterial colonization. As the airways are humidified, bacterial tracheitis onset is thus more likely [25].
Ventilator-associated bacterial tracheobronchitis (VABT) is characterized by the same criteria as ventilator-associated bacterial pneumonia (VABP): at least two of the following signs after 48 hours minimum on mechanical ventilation (MV): (1) temperature < 36 °C or ≥ 38.3 °C, (2) leukocytes < 5000 or ≥ 10,000/mm3, (3) onset or increase in purulent tracheal secretions, but without any radiological pulmonary infiltrate [26]. Distinguishing VABT from VABP depends on the diagnostic limitations of the chest x-ray. A positive culture of protected tracheal aspiration in adults (106 CFU/mL) is often used, just like bacteriuria during cystitis and pyelonephritis [25]. Despite this high and constant bacterial colonization, bacterial tracheitis onset remains rare. The incidence of VABT has been evaluated at 10.2/1000 days of MV in the most recent prospective study of 2960 patients on MV, i.e. 11% of patients. This incidence is similar to that of VABP [27]. VABT onset is associated with a prolonged hospital stay (median of 21 versus 12 days, P < 0.0001) [27].
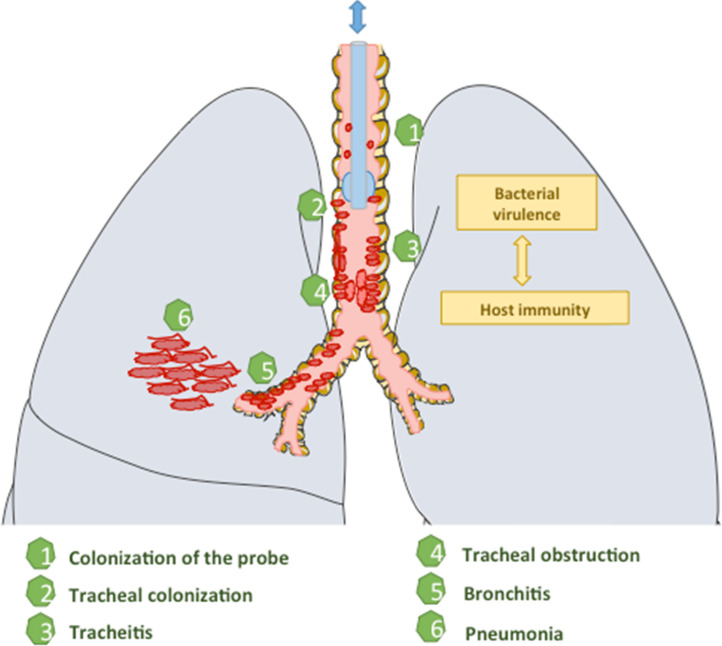
A continuum is very likely between tracheal colonization, tracheitis, bronchitis, and pneumonia (Fig. 1 ). Postmortem studies highlight a continuum between bronchitis and pneumonia in patients on MV [28]. For some patients, VABT is temporary as it is in-between bacterial colonization of the trachea and VABP. This pattern is similar to that of bacteriuria/cystitis/pyelonephritis [25]. Distinguishing a colonization from an infection is therefore very difficult. Warning signs are fever, hypoxemia, respiratory function deterioration, increased production of secretions, and leukocytosis with neutrophils. Tracheoscopy must be performed as often as possible. The procedure is easier in patients on life-support. Tracheoscopy is the reference examination to establish the diagnosis of tracheitis. It helps in restricting antibiotic prescription to tracheitis patients. The microbiological documentation helps correct the antibiotic spectrum of activity (22% of inappropriate empirical antibiotic therapies in the prospective study of Martin-Loeches et al. with an excessive mortality in this subgroup [27]). It also helps in antibiotic de-escalation whenever possible. For patients presenting with VABP, a diagnostic strategy based on ATP results is similar to a strategy based on bronchoalveolar lavage (BAL) in terms of clinical outcome [29]. Repeated ATP testing in patients on MV helps in precisely determining the microbiological origin of any subsequent VABP and in the early introduction of an adequate antibiotic therapy, with a very good correlation with BAL results [25], [29]. Repeated ATP quantitative microbiological examination is widely used to screen for resistant bacteria responsible for colonization. The aim is to tailor at best the empirical antibiotic therapy in case of VABP onset. VABTs are frequently caused by Gram-negative bacilli (75%) and by multiple bacteria (22%). P. aeruginosa is the most frequent, followed by S. aureus and Acinetobacter baumannii [30], [31] (Table 1).
Fig. 1.
Origins and consequences of ventilator-associated tracheitis.
Déterminants et conséquences de la trachéite acquise sous ventilation.
A chest x-ray is always performed to look for an associated alveolar pneumonia. Distinguishing a new pulmonary infiltrate from congestive signs or atelectasis with a chest x-ray is often difficult. Craven et al. suggested to perform a chest CT scan [25], but this examination is not always performed due to logistic reasons, irradiation, and the need to treat VABPs and VABTs with antibiotics.
The spectrum of the empirical antibiotic therapy must take into consideration Gram-positive cocci and nosocomial Gram-negative bacilli (Table 1). It must take into consideration prior tracheal microbiological documentations and be tailored to the tracheal culture results during the related episode. Knowing whether to treat a VABT has been extensively discussed. It is estimated that a third of VABTs progress into VABP. The case fatality rate is significantly higher at the end of VABP (40%) than VABT (29%), while the case fatality rate in the absence of lower respiratory tract infection was evaluated at 30% in the largest prospective study of patients on MV in the ICU [27]. Two randomized controlled studies highlighted the benefit of an intravenous and/or nebulized antibiotic therapy during VABT in adults to reduce the risk of VABP onset [30], [32]. The proof of concept was extrapolated in children, in the absence of data in this population. The authors of a study of children aged below 18 years did not observe any association between a prolonged treatment (≥ 7 days) and the reduction of VABP onset as compared with a short treatment [31]. A randomized placebo-controlled study evaluating the impact of the addition of a nebulized antibiotic therapy for VABT or VABP on the reduction of ventilation duration and hospitalization in the ICU is currently ongoing. Treatment of VABT therefore seems to be a reasonable strategy to improve patient outcome, as suggested by a meta-analysis [33]. The authors of a before/after study evaluating the impact of a multidisciplinary strategy aiming at reducing the incidence of respiratory tract infections on MV (elevate head of the bed to at least 30°, oral care with chlorhexidine, early extubation, healthcare workers’ hand hygiene) observed a reduced incidence of VABT from 3.9 cases to 1.8 cases per 1000 days of ventilation (P = 0.04) [34]. VABT management may be complicated by tracheal stenosis [35].
3.4. Particular situations
3.4.1. Tracheal aspergillosis
Tracheobronchitis aspergillosis (TA) is one of the least common presentations of invasive aspergillosis. It was evaluated at 8% of cases in a postmortem study [36]. A literature review retrieved 156 case patients of TA between 1985 and 2011. Nevertheless, the growing increase in the number of immunocompromised patients will probably lead to an increased incidence of this infection. The prescription of a corticosteroid therapy was observed in 71.8% of these reported case patients, and the infection often occurred in patients who had undergone organ transplantation (44.2%) or bone marrow transplant (8.3%), who presented with solid cancer (13.5%), or hemopathy (21.2%) [37]. AIDS led to the first reports of TA [38], but this predisposition is now rarely responsible for the infection since the launch of antiretrovirals. Most of these patients present with other associated predispositions (neutropenia, corticosteroids, etc.) [37]. Patients who underwent lung or heart transplants are at high risk of aspergillosis at the anastomotic site between the recipient's trachea and that of the host [39]. TA may also be a complication of active tuberculosis [37] or occur following post-tuberculosis stenosis [40]. Isolated tracheal involvement is rarer [41].
In 1995, Denning distinguished three presentations: pseudomembranous, ulcerative, and obstructive [42]. However, the coexistence of various presentations is frequently observed and several authors argued that they correspond to the various stages of a single process [39], [43]. The difficulty lies in distinguishing invasive presentations from simple colonization. The immunosuppression level is a major criterion, but endoscopy is required to better characterize the impairment. Wu et al. suggested a 4-category functional classification:
-
•
superficial infiltration;
-
•
involvement of the whole wall;
-
•
≥ 50% airway occlusion (by a pseudomembrane, a granuloma, a necrotic tissue);
-
•
mixed involvement [43].
Cough, fever, and dyspnea are the most frequent symptoms. Hemoptysis is reported in 11.5% of cases, and more frequently in immunocompetent patients. According to several authors, unilateral wheeze and stridor in neutropenic patients must lead physicians to suspect the diagnosis, but these symptoms are only observed in a quarter of patients [37]. Diagnosis is often delayed because of the non-specific presentation and because of the absence of radiological pulmonary abnormalities at the early stages of the infection. The CT scan results are normal in half of cases, and show non-specific abnormalities in a third of cases. CT scan is associated with better results than the x-ray [37] and must always be performed at the initial assessment and as part of treatment follow-up.
Tracheal samples must be carefully taken: ATP for microbiological documentation and as often as possible tracheal biopsy to better characterize the invasive nature of the infection [37]. Aspergillus fumigatus is most often isolated (74%), followed by Aspergillus flavus (11%) and Aspergillus niger (5%) [37]. The authors of the study of 156 TA patients reported that galactomannan level was assessed in only 10 patients; it was elevated in 6 of them and not only in neutropenic patients. Galactomannan level assessment is probably a useful diagnostic tool but it cannot rule out the aspergillosis origin in case of negativity [37]. Its level in tracheal secretions deserves to be assessed.
A first-line treatment with voriconazole is still recommended by the Infectious Diseases Society of America (IDSA) (grade B-II) [44]. Physicians must strive to reduce the immunosuppression. Amphotericin B aerosols could be beneficial as they would deliver high doses of polyene in the infected site, but this approach has never been evaluated [44].
Life-threatening complications are massive hemoptysis due to pulmonary vascular invasion by hyphae, and obstruction due to the accumulation of pseudomembranes of necrotic deposits. Independent risk factors for death are neutropenia and coexistence of acute respiratory distress syndrome [37].
3.4.2. Tuberculous tracheitis
First described in 1968, tracheobronchitis tuberculosis accounted for 42% of pulmonary tuberculosis (PTB) in a study of 1000 autopsies performed before the launch of combination therapies. This incidence is decreasing sharply: 10–36.8% of PTB cases in the oldest studies and 1% in the most recent study (1998–2000) of PTB patients who benefited from bronchoscopy [45], [46].
Isolated tracheal involvement is rare. It is diagnosed in the presence of stridor and persistent cough, and reveals an already advanced infection. It is usually associated with a pulmonary and bronchial involvement. The pathophysiological mechanism is still unknown, but the intratracheal infection by dissemination from a pulmonary localization remains the most probable hypothesis. Dissemination through the lymphatic route, from a pulmonary localization, or through the erosion of a cyst in the tracheal lumen, or even through the hematogenous route are being discussed [45]. The infection first manifests with an erythema and an edema with submucosal lymphocytic infiltrates followed by the formation of a tubercle. Extensive granulation of the tissue destroys and replaces the mucous and submucous membrane, and results in extensive fibrosis responsible for stenosis of the tracheobronchial tract [45]. Women seem to be more affected than men, probably because they spit less often than men due to cultural and social reasons [47], [48].
Clinically speaking, the presence of a productive cough, wheezing, and dyspnea in the absence of a history of asthma must lead physicians to suspect a tracheobronchial infection of tuberculosis origin when patients present with risk factors [47]. X-rays highlight the pulmonary parenchymatous involvement but rarely the tracheal lesions. The scan is far better to detect these lesions. Bronchoscopy is the reference examination for diagnosis and treatment follow-up. The most common presentations are mucosal granulations covered by caseous deposits and mucosal ulcerations [47]. These lesions lead to stenosis, that may persist or even worsen following effective treatment of the infection [48].
The corticosteroid therapy associated with the antituberculosis treatment could prevent the deterioration of the tracheobronchial stenosis [48], but its use remains controversial. A surgical or intrabronchial procedure can be suggested to patients presenting with a persistent symptomatic stenosis despite medical treatment [47].
3.4.3. Tracheal infection during pertussis
Coughing fits during pertussis are caused by airway inflammation induced by the bacterium itself, but mainly by several toxins such as the cytotracheal toxin that destroys, together with lipopolysaccharides (LPS), the mechanism of ciliary clearance and prevents its reparation [49]. Toxinic involvement during this infection triggers an inflammation of the whole airways. One should thus not speak about “pertussis tracheitis”. Nevertheless, postmortem studies confirm the key role of the trachea in the natural history of pertussis [50]. The authors of a prospective study reported that among 54 adults presenting with chronic laryngotracheitis, 24% had elevated IgA and IgG levels and 15% had elevated anti-toxin pertussis IgM levels [51].
3.4.4. Herpes virus-associated tracheitis
Herpes tracheobronchitis has already been reported, but isolated tracheal involvement has rarely been described [52]. It is most often due to a reactivation of the vagus nerve node or of the trigeminal nerve, but an associated stomatitis is usually observed with this latter specific case. The infection has mainly been reported in immunocompromised patients, in burn units, and in patients with prolonged tracheal intubation [52]. Most patients presented with persistent bronchospasm, sometimes with fever, despite conventional treatments (bronchodilators, corticosteroids) and one or several subsequent antibiotic therapies [52], [53]. The diagnosis may be suspected when ulcerative, vesicular, membranous, and/or hemorrhagic tracheitis is observed. Confirmation may be obtained with the presence of a cytopathogenic effect or by detecting the virus in tracheal secretions using culture or molecular biology techniques [52]. Bacterial coinfections are frequent [52]. The outcome is usually positive with acyclovir [52], [53].
Prolonged MV fosters herpes reactivations and herpes laryngotracheitis, which is a poor prognosis factor in this specific case [53], [54]. Confirming that HSV is the primum movens of the respiratory tract infection is difficult. HSV is rather a secondary disruptive factor, facilitated by corticosteroid therapies or even the consequence of the temporary immunodeficiency that follows a viral or bacterial infection. This hypothesis is increasingly supported by the presence of viral reactivations during sepsis.
Cytomegalovirus has sometimes, even though rarely, been responsible for tracheobronchial infection during HIV infections at AIDS stage [55]. A VZV-associated tracheal vesicular infection has also been reported during disseminated varicella in a 33-year-old immunocompetent patient [56].
3.5. Rarely reported cases
3.5.1. Other bacteria
A dozen of tracheal or laryngeal actinomycosis cases have been reported. Some were responsible for tracheal obstructions that required tracheostomy and prolonged penicillin antibiotic therapy [46].
Syphilitic gumma is rarely localized in the trachea [57].
Scleroma, an infectious granulomatous infection (Klebsiella rhinoscleromatis), is mainly localized in nasal fossae but may reach the trachea or be exclusively localized in the laryngotrachea – even though rarely – and lead to tracheal stenosis [58].
Bacillus cereus was implicated only once in an immunocompromised female patient [59].
Laryngeal and tracheobronchial infection during diphtheria is rare. It might be a primary infection or an infection extension from a pharyngeal localization. The trachea and bronchial tubes are covered with edema and pseudomembranes which are responsible for airway obstruction, cyanosis, and suffocation episodes in infected patients [60].
Another corynebacterium, Corynebacterium pseudodiphtheriticum, has been responsible for necrotizing tracheitis [61].
3.5.2. Other respiratory mycoses
Airborne mycoses mainly present as pulmonary infections, more rarely as bronchial infections, and rarely as isolated tracheitis.
Tracheal infection has rarely been reported during cryptococcosis. An isolated infection has been reported once as an obstructive tracheal mass [62].
Tracheal infection during mucormycosis is often associated with a laryngeal and/or pulmonary infection, sometimes isolated. It is mainly reported in diabetic patients, and has once been reported following H1N1 influenza infection. Bronchoscopy usually reveals necrotic tissue and a destructive infection. Liposomal amphotericin B and surgery are the backbone of the initial treatment [63]. Posaconazole is used as a treatment switch or in case of contraindication to amphotericin B. Amphotericin B aerosols could be associated, although never assessed.
Rare cases of tracheal infections have been reported during histoplasmosis [64], penicilliosis [65], fusariosis [66], coccidioidomycosis [67], paracoccidioidomycosis [68], and blastomycosis [69]. Candida is frequently isolated from respiratory samples and considered a colonizing fungus [70].
4. Conclusion
The trachea plays a major role in the development of lower respiratory tract infections. During viral infection, the trachea contributes to the bacterial superinfection and the difficulty lies in recognizing the progression into bacterial tracheitis in children as the morbidity and mortality remain high. During MV or tracheostomy, distinguishing bacterial tracheitis from simple colonization is difficult as tracheitis evolving into acquired pneumonia is frequent and associated with an excessive mortality rate. Non-specific clinical signs and difficulties in accessing the organ complicate the management. Persistent stridor and hoarse cough are suggestive signs. An endoscopic examination must be performed in patients presenting with severe symptoms, persistent obstruction, or in patients on MV. The endoscopic examination helps in establishing the diagnosis and in collecting adequate samples. Indeed, the etiological spectrum and pathophysiological processes are wide. Diagnostic delay may lead in the long-term to tracheal stenosis, which management is complex.
Contribution of authors
M.B. and P.B. performed the literature analysis and wrote the article.
N.F., P.B., and P.C. reviewed the article.
L.P. is the project initiator and coordinated it until the submission of the article.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Hitier M., Loälec M., Patron V., Edy E., Moreau S. [Trachée : anatomie, physiologie, endoscopie et imagerie] EMC Oto-rhino-laryngologie. 2013;8:1–18. [Google Scholar]
- 2.Denny F.W., Murphy T.F., Clyde W.A., Collier A.M., Henderson F.W. Croup: an 11-year study in a pediatric practice. Pediatrics. 1983;71:871–876. [PubMed] [Google Scholar]
- 3.Stroud R.H., Friedman N.R. An update on inflammatory disorders of the pediatric airway: epiglottitis, croup, and tracheitis. Am J Otolaryngol. 2001;22:268–275. doi: 10.1053/ajot.2001.24825. [DOI] [PubMed] [Google Scholar]
- 4.Bjornson C.L., Johnson D.W. Croup. Lancet. 2008;371:329–339. doi: 10.1016/S0140-6736(08)60170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong M.C., Chu M.C., Leighton S.E., van Hasselt C.A. Adult croup. Chest. 1996;109:1659–1662. doi: 10.1378/chest.109.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yedururi S., Guillerman R.P., Chung T., Braverman R.M., Dishop M.K., Giannoni C.M. Multimodality imaging of tracheobronchial disorders in children. Radiogr Rev Publ Radiol Soc N Am Inc. 2008;28(3):e29. doi: 10.1148/rg.e29. [DOI] [PubMed] [Google Scholar]
- 7.Lee E.Y., Restrepo R., Dillman J.R., Ridge C.A., Hammer M.R., Boiselle P.M. Imaging evaluation of pediatric trachea and bronchi: systematic review and updates. Semin Roentgenol. 2012;47:182–196. doi: 10.1053/j.ro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Jones R., Santos J.I., Overall J.C. Bacterial tracheitis. JAMA. 1979;242:721–726. [PubMed] [Google Scholar]
- 9.Kasian G.F., Bingham W.T., Steinberg J., Ninan A., Sankaran K., Oman-Ganes L. Bacterial tracheitis in children. CMAJ. 1989;140:46–50. [PMC free article] [PubMed] [Google Scholar]
- 10.Marx A., Török T.J., Holman R.C., Clarke M.J., Anderson L.J. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997;176:1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 11.Ross L.A., Mason W.H., Lanson J., Deakers T.W., Newth C.J. Laryngotracheobronchitis as a complication of measles during an urban epidemic. J Pediatr. 1992;121:511–515. doi: 10.1016/s0022-3476(05)81136-9. [DOI] [PubMed] [Google Scholar]
- 12.Tebruegge M., Pantazidou A., Thorburn K., Riordan A., Round J., De Munter C. Bacterial tracheitis: a multi-centre perspective. Scand J Infect Dis. 2009;41:548–557. doi: 10.1080/00365540902913478. [DOI] [PubMed] [Google Scholar]
- 13.Eckel H.E., Widemann B., Damm M., Roth B. Airway endoscopy in the diagnosis and treatment of bacterial tracheitis in children. Int J Pediatr Otorhinolaryngol. 1993;27:147–157. doi: 10.1016/0165-5876(93)90130-u. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein T., Brilli R., Jacobs B. Is bacterial tracheitis changing? A 14-month experience in a pediatric intensive care unit. Clin Infect Dis. 1998;27:458–462. doi: 10.1086/514681. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mutairi B., Kirk V. Bacterial tracheitis in children: approach to diagnosis and treatment. Paediatr Child Health. 2004;9:25–30. doi: 10.1093/pch/9.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyer C.M., Nuesslein T.G., Jung D., Peters S.A., Lemburg S.P., Rieger C.H.L. Tracheobronchial anomalies and stenoses: detection with low-dose multidetector CT with virtual tracheobronchoscopy – comparison with flexible tracheobronchoscopy. Radiology. 2007;242:542–549. doi: 10.1148/radiol.2422060153. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson J.D., Maltby C.C. Bacterial tracheitis in children. J Otolaryngol. 1989;18:101–104. [PubMed] [Google Scholar]
- 18.Hall C.B., Long C.E., Schnabel K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 19.Wu C., Cheng X., Wang X., Lv X., Yang F., Liu T. Clinical and molecular characteristics of the 2009 pandemic influenza H1N1 infection with severe or fatal disease from 2009 to 2011 in Shenzhen, China. J Med Virol. 2013;85:405–412. doi: 10.1002/jmv.23295. [DOI] [PubMed] [Google Scholar]
- 20.Gill J.R., Sheng Z.-M., Ely S.F., Guinee D.G., Beasley M.B., Suh J. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134:235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiken T., Holmes E.C., McCauley J., Rimmelzwaan G.F., Williams C.S., Grenfell B.T. Host species barriers to influenza virus infections. Science. 2006;312:394–397. doi: 10.1126/science.1122818. [DOI] [PubMed] [Google Scholar]
- 22.Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlid R., Andersson G., Frostell C.G., Jörbeck H.J., Ortqvist A.B. Respiratory tract colonization and infection in patients with chronic tracheostomy. A one-year study in patients living at home. Am J Respir Crit Care Med. 1996;154:124–129. doi: 10.1164/ajrccm.154.1.8680667. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki Y., Hirai K., Honda T. Pseudomembranous tracheobronchitis caused by methicillin-resistant Staphylococcus aureus. Scand J Infect Dis. 2002;34:211–213. doi: 10.1080/00365540110077083. [DOI] [PubMed] [Google Scholar]
- 25.Craven D.E., Hjalmarson K.I. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–S66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 26.Niederman M.S. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis. 2010;51(Suppl 1):S12–S17. doi: 10.1086/653035. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Loeches I., Povoa P., Rodríguez A., Curcio D., Suarez D., Mira J.-P. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3:859–868. doi: 10.1016/S2213-2600(15)00326-4. [DOI] [PubMed] [Google Scholar]
- 28.Rouby J.J., Martin De Lassale E., Poete P., Nicolas M.H., Bodin L., Jarlier V. Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. Am Rev Respir Dis. 1992;146:1059–1066. doi: 10.1164/ajrccm/146.4.1059. [DOI] [PubMed] [Google Scholar]
- 29.Canadian Critical Care Trials Group A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619–2630. doi: 10.1056/NEJMoa052904. [DOI] [PubMed] [Google Scholar]
- 30.Palmer L.B., Smaldone G.C., Chen J.J., Baram D., Duan T., Monteforte M. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–2013. doi: 10.1097/CCM.0b013e31817c0f9e. [DOI] [PubMed] [Google Scholar]
- 31.Tamma P.D., Turnbull A.E., Milstone A.M., Lehmann C.U., Sydnor E.R.M., Cosgrove S.E. Ventilator-associated tracheitis in children: does antibiotic duration matter? Clin Infect Dis. 2011;52:1324–1331. doi: 10.1093/cid/cir203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nseir S., Favory R., Jozefowicz E., Decamps F., Dewavrin F., Brunin G. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care Lond Engl. 2008;12:R62. doi: 10.1186/cc6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craven D.E., Hudcova J., Lei Y. Ventilator-associated tracheobronchitis: pre-emptive, appropriate antibiotic therapy recommended. Crit Care. 2014;18:627. doi: 10.1186/s13054-014-0627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muszynski J.A., Sartori J., Steele L., Frost R., Wang W., Khan N. Multidisciplinary quality improvement initiative to reduce ventilator-associated tracheobronchitis in the PICU. Pediatr Crit Care Med. 2013;14:533–538. doi: 10.1097/PCC.0b013e31828a897f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chechani V., Vasudevan V.P., Kamholz S.L. Necrotizing tracheobronchitis: complication of mechanical ventilation in an adult. South Med J. 1991;84:271–273. [PubMed] [Google Scholar]
- 36.Young R.C., Bennett J.E., Vogel C.L., Carbone P.P., DeVita V.T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970;49:147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Ruiz M., Silva J.T., San-Juan R., de Dios B., García-Luján R., López-Medrano F. Aspergillus tracheobronchitis: report of 8 cases and review of the literature. Medicine (Baltimore) 2012;91:261–273. doi: 10.1097/MD.0b013e31826c2ccf. [DOI] [PubMed] [Google Scholar]
- 38.Kemper C.A., Hostetler J.S., Follansbee S.E., Ruane P., Covington D., Leong S.S. Ulcerative and plaque-like tracheobronchitis due to infection with Aspergillus in patients with AIDS. Clin Infect Dis. 1993;17:344–352. doi: 10.1093/clinids/17.3.344. [DOI] [PubMed] [Google Scholar]
- 39.Kramer M.R., Denning D.W., Marshall S.E., Ross D.J., Berry G., Lewiston N.J. Ulcerative tracheobronchitis after lung transplantation. A new form of invasive aspergillosis. Am Rev Respir Dis. 1991;144:552–556. doi: 10.1164/ajrccm/144.3_Pt_1.552. [DOI] [PubMed] [Google Scholar]
- 40.Pornsuriyasak P., Murgu S., Colt H. Pseudomembranous aspergillus tracheobronchitis superimposed on post-tuberculosis tracheal stenosis. Respirol Carlton Vic. 2009;14:144–147. doi: 10.1111/j.1440-1843.2008.01389.x. [DOI] [PubMed] [Google Scholar]
- 41.Grosu H.B., Bashoura L., Ost D., Ordonez N.G., Faiz S.A. Critical airway obstruction due to pseudomembranous Aspergillus tracheitis. Am J Respir Crit Care Med. 2014;190:e65–e66. doi: 10.1164/rccm.201405-0895IM. [DOI] [PubMed] [Google Scholar]
- 42.Denning D.W. Commentary: unusual manifestations of aspergillosis. Thorax. 1995;50:812–813. doi: 10.1136/thx.50.7.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu N., Huang Y., Li Q., Bai C., Huang H.-D., Yao X.-P. Isolated invasive Aspergillus tracheobronchitis: a clinical study of 19 cases. Clin Microbiol Infect. 2010;16:689–695. doi: 10.1111/j.1469-0691.2009.02923.x. [DOI] [PubMed] [Google Scholar]
- 44.Walsh T.J., Anaissie E.J., Denning D.W., Herbrecht R., Kontoyiannis D.P., Marr K.A. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y., Lee K.S., Yoon J.H., Chung M.P., Kim H., Kwon O.J. Tuberculosis of the trachea and main bronchi: CT findings in 17 patients. AJR Am J Roentgenol. 1997;168:1051–1056. doi: 10.2214/ajr.168.4.9124114. [DOI] [PubMed] [Google Scholar]
- 46.Hagan M.E., Klotz S.A., Bartholomew W., Cherian R., McGregor D. Actinomycosis of the trachea with acute tracheal obstruction. Clin Infect Dis. 1996;22:1126–1127. doi: 10.1093/clinids/22.6.1126. [DOI] [PubMed] [Google Scholar]
- 47.Le Huu L., Phan Thanh H., Hoeffel C.C. [Active tracheo-bronchial caseous tuberculosis. Ten patients] Rev Pneumol Clin. 2001;57:289–295. [PubMed] [Google Scholar]
- 48.Chung H.S., Lee J.H. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000;117:385–392. doi: 10.1378/chest.117.2.385. [DOI] [PubMed] [Google Scholar]
- 49.Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paddock C.D., Sanden G.N., Cherry J.D., Gal A.A., Langston C., Tatti K.M. Pathology and pathogenesis of fatal Bordetella pertussis infection in infants. Clin Infect Dis. 2008;47(3):328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 51.Beaver M.E., Karow C.M. Incidence of seropositivity to bordetella pertussis and mycoplasma pneumoniae infection in patients with chronic laryngotracheitis. Laryngoscope. 2009;119:1839–1843. doi: 10.1002/lary.20535. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez-Uria G., Surinach J.M., Ventura A., de la Rosa D., de Gracia J., Fernandez-Sevilla T. Herpetic tracheitis and polybacterial pneumonia in an immunocompetent young man is herpes tracheitis involved in the pathogenesis of bacterial pneumonia? J Clin Virol. 2008;41:164–165. doi: 10.1016/j.jcv.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Sherry M.K., Klainer A.S., Wolff M., Gerhard H. Herpetic tracheobronchitis. Ann Intern Med. 1988;109:229–233. doi: 10.7326/0003-4819-109-3-229. [DOI] [PubMed] [Google Scholar]
- 54.Engelmann I., Gottlieb J., Meier A., Sohr D., Ruhparwar A., Henke-Gendo C. Clinical relevance of and risk factors for HSV-related tracheobronchitis or pneumonia: results of an outbreak investigation. Crit Care. 2007;11:R119. doi: 10.1186/cc6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace J.M., Hannah J. Cytomegalovirus pneumonitis in patients with AIDS. Findings in an autopsy series. Chest. 1987;92:198–203. doi: 10.1378/chest.92.2.198. [DOI] [PubMed] [Google Scholar]
- 56.Aichaouia C., Abada D., Moatamri Z., Hadaoui A.B., M’hamdi S., Khadraoui M. [Endobronchial chicken pox] Med Mal Infect. 2010;40:427–428. doi: 10.1016/j.medmal.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Lucas R. Case of Syphilitic Gumma situated in the trachea successfully treated by large doses of iodide of potassium. Br Med J. 1887;2:1378. doi: 10.1136/bmj.2.1408.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrak L., Maslout A., Benosmane A. [Tracheal scleroma and rhinoscleroma: a case report] Rev Pneumol Clin. 2007;63:115–118. doi: 10.1016/s0761-8417(07)90112-1. [DOI] [PubMed] [Google Scholar]
- 59.Strauss R., Mueller A., Wehler M., Neureiter D., Fischer E., Gramatzki M. Pseudomembranous tracheobronchitis due to Bacillus cereus. Clin Infect Dis. 2001;33:E39–E41. doi: 10.1086/322674. [DOI] [PubMed] [Google Scholar]
- 60.Hadfield T.L., McEvoy P., Polotsky Y., Tzinserling V.A., Yakovlev A.A. The pathology of diphtheria. J Infect Dis. 2000;181(Suppl 1):S116–S120. doi: 10.1086/315551. [DOI] [PubMed] [Google Scholar]
- 61.Guerrero J., Mallur P., Folch E., Keyes C., Stillman I.E., Gangadharan S.P. Necrotizing tracheitis secondary to corynebacterium species presenting with central airway obstruction. Respir Care. 2014;59:e5–e8. doi: 10.4187/respcare.02150. [DOI] [PubMed] [Google Scholar]
- 62.Sun L., Chen H., Shao C., Song Y., Bai C. Pulmonary cryptococcosis with trachea wall invasion in an immunocompetent patient: a case report and literature review. Respir Int Rev Thorac Dis. 2014;87:324–328. doi: 10.1159/000357715. [DOI] [PubMed] [Google Scholar]
- 63.Mohindra S., Gupta B., Gupta K., Bal A. Tracheal mucormycosis pneumonia: a rare clinical presentation. Respir Care. 2014;59:e178–e181. doi: 10.4187/respcare.03174. [DOI] [PubMed] [Google Scholar]
- 64.Youness H., Michel R.G., Pitha J.V., Jones K.R., Kinasewitz G.T. Tracheal and endobronchial involvement in disseminated histoplasmosis: a case report. Chest. 2009;136:1650–1653. doi: 10.1378/chest.09-0236. [DOI] [PubMed] [Google Scholar]
- 65.Ye F., Luo Q., Zhou Y., Xie J., Zeng Q., Chen G. Disseminated penicilliosis marneffei in immunocompetent patients: a report of two cases. Indian J Med Microbiol. 2015;33:161–165. doi: 10.4103/0255-0857.148433. [DOI] [PubMed] [Google Scholar]
- 66.Peltroche-Llacsahuanga H., Manegold E., Kroll G., Haase G. Case report. Pathohistological findings in a clinical case of disseminated infection with Fusarium oxysporum. Mycoses. 2000;43:367–372. doi: 10.1046/j.1439-0507.2000.00590.x. [DOI] [PubMed] [Google Scholar]
- 67.Polesky A., Kirsch C.M., Snyder L.S., LoBue P., Kagawa F.T., Dykstra B.J. Airway coccidioidomycosis – report of cases and review. Clin Infect Dis. 1999;28:1273–1280. doi: 10.1086/514778. [DOI] [PubMed] [Google Scholar]
- 68.Marchiori E., Escuissato D.L., Souza A.S., Barillo J.L., Warszawiak D., de Souza A.S. Computed tomography findings in patients with tracheal paracoccidioidomycosis. J Comput Assist Tomogr. 2008;32:788–791. doi: 10.1097/RCT.0b013e3181506752. [DOI] [PubMed] [Google Scholar]
- 69.Kaufman J. Tracheal blastomycosis. Chest. 1988;93:424–425. doi: 10.1378/chest.93.2.424. [DOI] [PubMed] [Google Scholar]
- 70.Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L. Executive summary: clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]