Fig. 3.
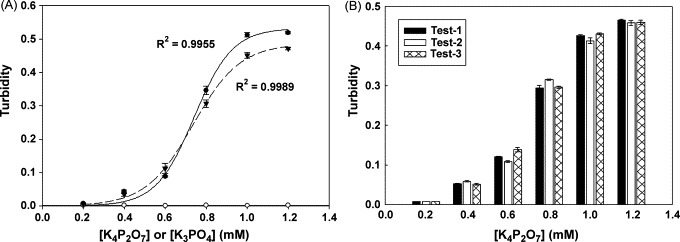
To evaluate the efficiency and reproducibility of detecting the turbidity in the base apparatus by using chemical simulation reaction. (A) The by-product production of the LAMP reaction can be synthesized by chemical reaction. The reaction reagent contains Mg2+ ion and P2O74− (filled color) or PO43− (opened color) ion in reaction buffer. After 60 min reaction at 65 °C, turbidity can be detected by spectrometer (filled circle) at 533 nm wavelength or base apparatus (filled triangle). The mean values (triplicate tests) of turbidity closely fit the sigmoid curves. (B) We demonstrate the results of the within and between run to exhibit the reproducibility and stability of the base apparatus for turbidity detection. In our experiment, three sets of triplicate tests (inter- and intra-assay) under six different concentrations of pyrophosphate ion are performed. It shows that the coefficient of variation (CV%) of results is less than 6% of turbidity detection.
