Fig. 1.
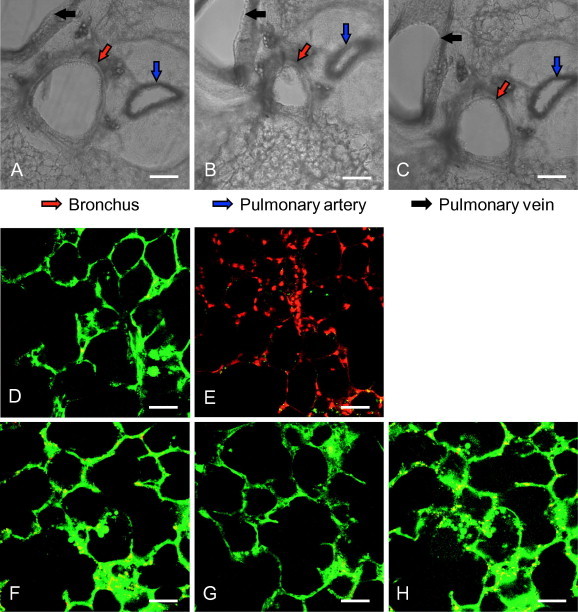
Mouse lung slices remain viable for at least 5 days ex vivo. (A–C) The 250-μm thick lung slices were prepared, and the bronchoconstriction was observed 5 days after preparation. The untreated slices (A) were incubated with 0.1 μM acetyl-ß-methylcholine chloride at 37 °C for 5 min (B) in a 24-well cell culture plate. Then, the drug was removed, lung slices were washed twice with PBS and incubated with fresh culture medium at 37 °C for 10 min (C). The images were captured using a Nikon’s Eclipse Ti inverted microscope. Red, blue and black arrows show the lung bronchus, pulmonary artery and pulmonary vein, respectively. Scale bar = 200 μm. (D–H) Live/dead staining assay. Lung slices were stained with Calcein-AM (1 μM) and Propidium Iodide (PI, 1 μg/ml) for 20 min at room temperature on days 1, 3 and 5 after preparation (lower panels, F–H). The middle panel shows a viable slice directly after preparation (D), and a slice with complete loss of activity (treated by 1% Triton-100) (E) for comparison. Calcein-AM and PI were used to simultaneously determine the live and dead cells. After being washed twice with PBS, images were taken using a Nikon multiphoton Confocal Microscope A1 MP+ with excitation at 800 nm, and an emission filter of 500/50 nm for Calcein-AM and 625/50 nm for PI. Scale bar = 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
