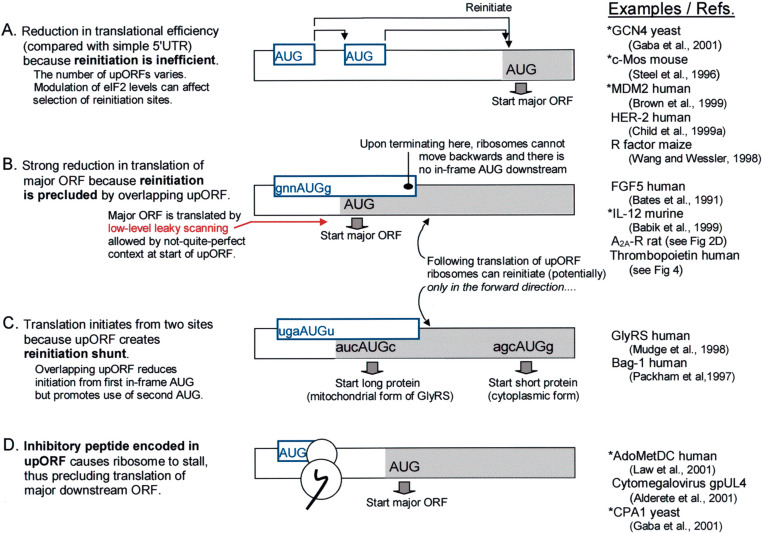
Fig. 3.
Small upstream ORFs in eukaryotic mRNAs function in various ways to modulate translation. Only the 5′ end of each mRNA is depicted. (A) The presence of upORFs forces translation of the major ORF to occur by a reinitiation mechanism, which is usually inefficient. The extent of inhibition depends on the number and arrangement of upORFs and whether the context flanking the upstream start codon(s) allows some escape via leaky scanning. (B) Because reinitiation can occur only in the forward direction, an overlapping upORF strongly impairs translation of the major ORF. (C) Whereas type B mRNAs have a single in-frame start codon which is bypassed due to the overlapping upORF, type C mRNAs initiate from two in-frame start codons; the upORF serves to divert some ribosomes to the downstream start site. The depicted sequence is a simplified representation of GlyRS mRNA (Mudge et al., 1998). Translation of Bag-1 mRNA can also be fitted to this pattern: the first start site is an in-frame CUG codon which produces the 50 kDa form of Bag-1; the next start site (AUG#1, out-of-frame) initiates a small upORF within which the first in-frame AUG codon (AUG#2) resides, and that AUG is thereby skipped; the 36 kDa form of Bag-1 is produced from AUG#3 which is accessed by reinitiation following translation of the small upORF (Packham et al., 1997). Some other mRNAs that use an upORF to dodge one AUG codon in favor of another are described elsewhere (Mittag et al., 1997, Sarrazin et al., 2000). Note that the reinitiation shunt as here defined adheres to the linear scanning mechanism, unlike a shunt postulated to operate with cauliflower mosaic virus mRNA (Ryabova et al., 2000). (D) The common feature of mRNAs that use mechanism D is inhibition of translation in cis by a peptide encoded within the upORF. The amino acid sequence of the inhibitory peptide is different in each case (Morris and Geballe, 2000). In the column at the far right, asterisks indicate examples in which the translational control mechanism is regulated, e.g. via a change in concentration of eIF2 (GCN4) or arginine (CPA1) or polyamines (AdoMetDC) or, more commonly, via an alternative promoter that generates a simpler form of mRNA devoid of upORFs (c-mos, MDM2, IL-12; see text for other examples).