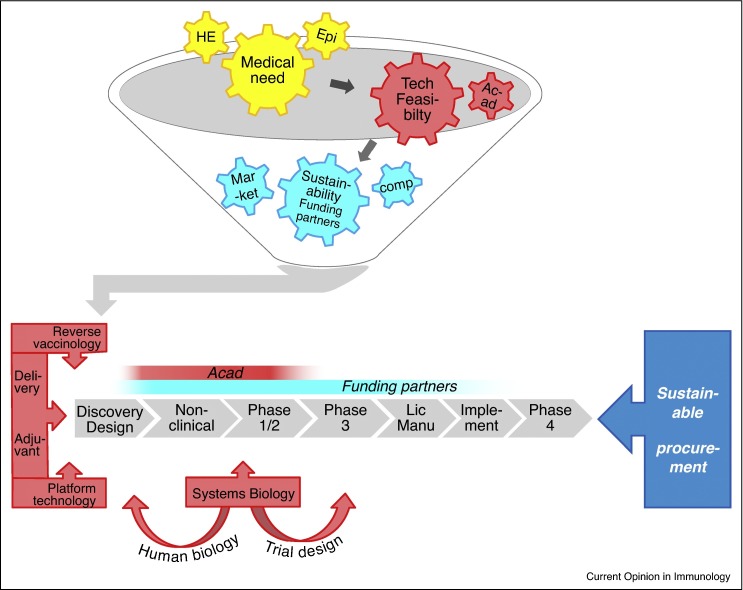
Figure 1.
Sustainable vaccine development and collaborations. Funnel: Guiding criteria for vaccine project prioritization are, first, the unmet medical need, as supported by health-economical (‘HE’) and epidemiological (‘Epi’) evaluations; second, technical feasibility, often benefitting from partnerships with academia (‘Acad’), and third, the sustainability of its development, which depends on the availability of funding partners for collaborative development, as well as on the competitive landscape (‘comp’) and the economic development status of the market for which the vaccine is intended (‘Market’). While the development of vaccines with a market that includes high-income countries is often predominantly industry-funded, trials evaluating vaccines for predominantly low-to-middle-income markets, or prepandemic vaccines, are typically co-funded by public–private partnerships including industry, governments and international non-governmental organizations. Bars: Red and blue bars indicate the development stages typically benefitting from involvement/support by academia and international funding organizations, respectively. Academic partners mostly contribute by providing immunological insights in late preclinical and Phase 1/2 clinical phases. Funding partners can provide support throughout the whole process, including the licensing (‘Lic’) phase, and the post-licensing phases comprising vaccine manufacturing (‘Manu’) and implementation (‘Implement’), for example the supply chain management support provided by the public–private partnership (mVacciNation). Post-marketing Phase 4 studies monitoring vaccine usage, adverse effects (pharmacovigilance), and long-term immunity are typically industry-funded. Red arrowed bars indicate the technologies used to guide antigen discovery and/or vaccine design (reverse vaccinology, delivery, adjuvants and platform technologies), while systems biology data, often generated in industry-academic partnerships, can guide during the discovery phase, as well as in later clinical phases, by supporting adaptive trial designs to expedite progression to Phase 3 clinical evaluations. Finally, strategies to manage the sustainable procurement of new vaccines, such as tiered pricing policies, will also majorly drive the vaccine development process.