Abstract
Purpose
Prospective single-center study to assess the sensitivity and clinical relevance of molecular testing for respiratory viruses in critically ill immunocompromised patients with acute respiratory failure (ARF).
Methods
100 consecutive critically ill immunocompromised patients with ARF in 2007–2009. Among them, 65 had hematologic malignancies (including 14 hematopoietic stem cell transplant recipients), 22 had iatrogenic immunosuppression, and 13 had solid malignancies. A multiplex molecular assay (MMA) was added to the usual battery of tests performed to look for causes of ARF.
Results
Nasopharyngeal aspirates and/or bronchoalveolar lavage fluid were tested for respiratory viruses using both the MMA and immunofluorescence. A virus was detected in 47 (47%) patients using the MMA and 8 (8%) patients using immunofluorescence (P = 0.006). MMA-positive and MMA-negative patients had similar clinical and radiographic presentations and were not significantly different for the use of ventilatory support (58% vs. 76%, P = 0.09), occurrence of shock (43% vs. 53%, P = 0.41), use of renal replacement therapy (26% vs. 23%, P = 0.92), SAPS II (35 [26–44] vs. 38 [27–50], P = 0.36), time spent in the ICU (6 vs. 7 days, P = 0.35), or ICU mortality (17% vs. 28%, P = 0.27). Using MMA, a virus was found in 6 of the 12 patients with no diagnosis at the end of the etiologic investigations.
Conclusions
In critically ill immunocompromised patients, an MMA was far more sensitive than immunofluorescence for respiratory virus detection. Patients with RVs detected in the respiratory tract had the same clinical characteristics and outcomes as other patients.
Keywords: Acute respiratory failure, Immunosuppression, Respiratory viruses, Polymerase chain reaction assay
Abbreviation: ARF, acute respiratory failure; BAL, bronchoalveolar lavage; CI, confidence interval; hMPV, human metapneumovirus; HSCT, hematopoietic stem cell transplantation; ICU, intensive care unit; IQR, interquartile range; MMA, multiplex molecular assay; MLPA, Multiplex Ligation-dependent Probe-Amplification; NA, not available; NPA, nasopharyngeal aspirate; OR, odds ratio; PCR, polymerase chain reaction; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, respiratory virus; SAPS II, simplified acute physiology score II
Introduction
Acute respiratory failure (ARF) occurs in up to 50% of patients with malignancies, who are then at high risk for death, particularly if they require mechanical ventilation.1, 2, 3, 4 Respiratory viruses (RVs) are detected in 10%–20% of these ARF episodes.5, 6 Together with the bacterial and fungal infections often seen in this setting, RV infections are potentially life-threatening in immunocompromised patients.7, 8, 9, 10, 11
Viral culture has long been considered the reference standard for diagnosing RV infection but usually takes several days to yield results. Antigen detection is faster but less sensitive.12 Molecular screening based on the polymerase chain reaction (PCR) is fast, more sensitive than earlier test methods, highly specific, and capable of detecting rhinoviruses and coronaviruses, which are missed by other tests.13 Molecular assays have been reported to improve the diagnostic yield compared with conventional methods during acute respiratory illnesses in patients with hematological malignancies.14, 15, 16, 17 However, they detect only nucleic acids, as opposed to live organisms, and their clinical relevance is therefore unclear. A positive molecular assay on a respiratory sample may indicate viral infection, colonization, or contamination.
The purpose of this study was to evaluate a multiplex molecular assay (MMA) comparatively with immunofluorescence for RV detection in immunocompromised patients admitted to intensive care unit (ICU) with ARF and to assess the clinical relevance of the MMA results.
Patients and methods
Immunocompromised patients with hypoxemic ARF admitted to our closed ICU in a teaching hospital from January 2007 to July 2009 were included prospectively in this cohort study. The MMA was the intervention. The institutional review board of the Clermont Ferrand teaching hospital approved this study and waived the need for informed consent.
Immunocompromised status was defined as presence of a disease or treatment known to impair the immune system, such as a hematological or metastatic solid malignancy or long-term corticosteroid therapy, immunosuppressive therapy, cytotoxic chemotherapy, and/or bone marrow or hematopoietic stem cell transplantation (HSCT). Patients infected with the human immunodeficiency virus were not included.
ARF was defined as a respiratory rate greater than 30 breaths per minute or respiratory distress symptoms or PaO2 on room air lower than 8 kPa or a need for ventilatory support.
The data reported in Table 1, Table 2, Table 3 were collected for each study patient. Simplified acute physiology score II (SAPS II), time in the ICU and vital status at ICU discharge were also collected.18
Table 1.
Baseline characteristics, symptoms, chest radiography patterns, need for life-supporting interventions, and outcomes of the 100 immunocompromised patients with acute respiratory failure and comparaison of patients with and without a positive MMA.
| Characteristics | Total | Positive MMA | Negative MMA | P value | OR (95%CI) |
|---|---|---|---|---|---|
| Patients | 100 | 47 | 53 | / | / |
| 65 (65%) | 29 (62%) | 36 (68%) | |||
| Male/Female | / | / | / | 0.66 | 0.76 (0.33–1.73)b |
| 35 (35%) | 18 (38%) | 17 (32%) | |||
| Agea | 60 (44–67) | 58 (41–67) | 60 (46–67) | 0.45 | 0.99 (0.97–1.01) |
| Community-acquired ARFc | 43 (43%) | 22 (47%) | 21 (40%) | 0.6 | 1.34 (0.61–2.97) |
| Immunosuppressants in the past month | 76 (76%) | 40 (85%) | 36 (68%) | 0.05 | 2.7 (1.0–7.25) |
| Corticosteroids in the past month | 39 (39%) | 21 (45%) | 18 (34%) | 0.36 | 1.6 (0.71–3.64) |
| Underlying disease | |||||
| Hematological malignancy | 65 (65%) | 29 (62%) | 36 (68%) | 0.66 | 0.76 (0.33–1.73) |
| Lymphoma | 28 (28%) | 14 (30%) | 14 (26%) | 0.88 | 1.18 (0.49–2.83) |
| Acute lymphocytic leukemia | 5 (5%) | 2 (4%) | 3 (6%) | 0.99 | 0.74 (0.12–4.64) |
| Acutemyelogenous leukemia | 16 (16%) | 6 (12%) | 10 (18%) | 0.58 | 0.63 (0.21–1.89) |
| Chronic lymphocytic leukemia | 4 (4%) | 1 (2%) | 3 (6%) | 0.7 | 0.36 (0.04–3.61) |
| Chronicmyelogenous leukemia | 4 (4%) | 1 (2%) | 3 (6%) | 0.7 | 0.36 (0.04–3.61) |
| Myeloma | 8 (8%) | 6 (12%) | 2 (4%) | 0.2 | 3.73 (0.72–19.5) |
| Myelodysplastic disorder | 1 (1%) | 0 (0%) | 1 (2%) | 0.99 | / |
| HSCT | 14 (14%) | 9 (19%) | 5 (9%) | 0.27 | 2.27 (0.7–7.35) |
| Iatrogenic immunosuppression | 22 (22%) | 14 (30%) | 8 (15%) | 0.13 | 2.39 (0.9–6.35) |
| Solid organ transplantation | 16 (16%) | 12 (26%) | 4 (7.5%) | 0.03 | 4.2 (1.25–14.1) |
| Autoimmune disorders | 6 (6%) | 2 (4%) | 4 (7.5%) | 0.79 | 0.54 (0.1–3.12) |
| Solid tumors | 13 (13%) | 4 (8%) | 9 (17%) | 0.34 | 0.46 (0.13–1.59) |
| Metastatic solid tumor | 7 (7%) | 2 (4%) | 5 (9%) | 0.54 | 0.43 (0.08–2.31) |
| Symptoms | |||||
| Fever | 61 (61%) | 31 (66%) | 30 (57%) | 0.45 | 1.49 (0.66–3.35) |
| Cough | 49 (49%) | 32 (68%) | 24 (46%) | 0.07 | 2.5 (0.99–6.1) |
| General symptomsd | 8 (8%) | 5 (11%) | 3 (6%) | 0.59 | 1.98 (0.35–8.8) |
| Purulent sputum | 14 (14%) | 8 (17%) | 6 (11%) | 0.6 | 1.61 (0.51–5.02) |
| Chest radiography patterns | |||||
| Normal | 11 (11%) | 7 (15%) | 4 (8%) | 0.39 | 2.1 (0.59–7.85) |
| Lobar alveolar consolidation | 16 (16%) | 9 (19%) | 7 (16%) | 0.59 | 1.56 (0.53–4.57) |
| Diffuse alveolar consolidation | 48 (48%) | 23 (49%) | 25 (47%) | 0.99 | 1.1 (0.49–2.36) |
| Interstitial involvement | 41 (41%) | 22 (47%) | 19 (36%) | 0.36 | 1.58 (0.71–3.51) |
| Pleural effusion | 2 (14%) | 2 (4%) | 0 (0%) | 0.53 | / |
| Life-supporting interventions and outcomes | |||||
| Mechanical ventilation | 67 (67%) | 27 (58%) | 40 (76%) | 0.09 | 0.44 (0.19–1.03) |
| Shock | 48 (48%) | 20 (43%) | 28 (53%) | 0.41 | 0.66 (0.3–1.46) |
| Renal replacement therapy | 24 (24%) | 12 (26%) | 12 (23%) | 0.92 | 1.17 (0.47–2.94) |
| SAPS II | 36 (27–46) | 35 (26–44) | 38 (27–50) | 0.36 | 0.99 (0.96–1.02) |
| Time spent in the ICUe | 7 (3–13) | 6 (2–13) | 7 (3–17) | 0.35 | 0.99 (0.96–1.02) |
| ICU mortality | 23 (23%) | 8 (17%) | 15 (28%) | 0.27 | 0.52 (0.2–1.37) |
Data are number (percent), unless otherwise stated. Results are from the univariate analysis.
ARF, acute respiratory failure; HSCT, human stem cell transplantation; ICU, intensive care unit; MMA, multiplex molecular assay; OR, odds ratio; 95%CI, 95% confidence interval; SAPS II: simplified acute physiology score II.
Years, median (interquartile range).
For men.
Episode of acute respiratory failure beginning outside the hospital.
General symptoms: asthenia, myalgia, and headache.
Days, median (interquartile range).
Table 2.
Results of immunofluorescence and a multiplex molecular assay for respiratory viruses.
| Variables | Immunofluorescence | MMA | P value | |
|---|---|---|---|---|
| Influenza | All | 2 | 14 | 0.012 |
| A | 2 | 11 | 0.0035 | |
| B | 0 | 3 | / | |
| RSV | 3 | 4 | 0.99 | |
| PIV | All | 1 | 6 | 0.063 |
| 1 | NA | 0 | / | |
| 2 | NA | 1 | / | |
| 3 | NA | 4 | / | |
| 4 | NA | 1 | / | |
| hMPV | 1 | 6 | 0.063 | |
| Adenovirus | 1 | 4 | 0.018 | |
| Rhinovirus | 0 | 15 | / | |
| Coronavirus | 0 | 3 | / | |
| All virus | 8 | 52 | / | |
| All patients | 8 (8%) | 47 (47%) | 0.006 | |
Data are number (percent). Results are from the univariate analysis.
MMA, multiplex molecular assay; hMPV, human metapneumovirus; NA, not available; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
Table 3.
Microorganisms, use of life-supporting interventions, and outcomes in patients with and without documented lung infections and in patients with and without a positive MMA for respiratory viruses.
| Total | Positive MMA | Negative MMA | P value | OR (95%CI) | |
|---|---|---|---|---|---|
| Documented lung infection | 48 | 30 | 18 | / | / |
| Clinically documented infection | 29 (60%) | 19 (63%) | 10 (56%) | 0.82 | 1.4 (0.42–4.5) |
| Microorganisms in respiratory specimensb | 19 (40%) | 11 (37%) | 8 (44%) | 0.59 | 0.72 (0.22–2 0.4) |
| Bacteria | 11 (23%) | 5 (17%) | 6 (33%) | / | / |
| Streptococcus pneumoniae | 2 (4%) | 1 (3%) | 1 (5%) | / | / |
| Staphylococcus aureus | 2 (4%) | 2 (7%) | 0 | / | / |
| Haemophilus influenzae | 1 (2%) | 0 | 1 (5%) | / | / |
| Enterobacteriaceae | 2 (4%) | 1 (3%) | 1 (5%) | / | / |
| Pseudomonas aeruginosa | 3 (7%) | 1 (3%) | 2 (11%) | / | / |
| Acinetobacter baumanii | 1 (2%) | 0 | 1 (5%) | / | / |
| Fungus | 8 (17%) | 6 (20%) | 2 (11%) | / | / |
| Pneumocystis jirovecii | 5 (10%) | 4 (13%) | 1 (5%) | / | / |
| Aspergillus sp. | 3 (7%) | 2 (7%) | 1 (5%) | / | / |
| Mechanical ventilation | 33 (69%) | 20 (66%) | 13 (72%) | 0.69 | 0.77 (0.21–2.8) |
| Shock | 30 (63%) | 17 (57%) | 13 (72%) | 0.29 | 0.5 (0.14–1.8) |
| Duration of shocka | 2 (0–3) | 1 (0–3) | 3 (0–4) | 0.56 | 0.93 (0.74–1.2) |
| Renal replacement therapy | 14 (29%) | 11 (37%) | 3 (17%) | 0.15 | 2.9 (0.68–12.3) |
| Time spent in the ICUa | 8 (4–16) | 8 (4–13) | 8 (3–17) | 0.81 | 0.99 (0.96–1.0) |
| ICU mortality | 13 (27%) | 7 (23%) | 6 (33%) | 0.45 | 0.61 (0.17–2.2) |
| Non-infectious lung disease | 52 | 17 | 35 | / | / |
| Clinically documented infection | 0 | 0 | 0 | / | / |
| Microorganisms identified in respiratory specimenb | 4 (8%) | 1 (6%) | 3 (9%) | 0.73 | 0.67 (0.06–6.9) |
| Bacteria | 3 (6%) | 1 (6%) | 3 (9%) | / | / |
| Coagulase-negative | 1 (2%) | 1 (6%) | 0 | / | / |
| Staphylococci | |||||
| Enterobacteriaceae | 1 (2%) | 0 | 1 (3%) | / | / |
| Pseudomonas aeruginosa | 1 (2%) | 0 | 1 (3%) | / | / |
| Cytomegalovirus | 1 (2%) | 0 | 1 (3%) | / | / |
| Mechanical ventilation | 34 (65%) | 7 (41%) | 27 (77%) | 0.013 | 0.21 (0.06–0.72) |
| Shock | 18 (35%) | 3 (18%) | 15 (43%) | 0.08 | 0.29 (0.07–1.2) |
| Duration of shocka | 0 (0–2) | 0 (0–0) | 0 (0–3) | 0.008 | / |
| Renal replacement therapy | 10 (19%) | 1 (6%) | 9 (26%) | 0.12 | 0.18 (0.02–1.6) |
| Time spent in the ICUa | 6 (3–10) | 4 (2–9) | 7 (3–11) | 0.07 | 0.91 (0.81–1.0) |
| ICU mortality | 10 (19%) | 1 (6%) | 9 (26%) | 0.12 | 0.18 (0.02–1.6) |
Data are number (percent), unless otherwise stated. Results are from the univariate analysis.
ICU, intensive care unit; MMA, multiplex molecular assay; OR, odds ratio; 95%CI, 95% confidence interval.
Days, median (interquartile range).
Any bacterial, viral, and/or fungal agent identified in a respiratory specimen.
All patients were investigated using a previously described diagnostic strategy that relies heavily on noninvasive tests.19 Most of the patients underwent noninvasive tests for infections, such as sputum examination for bacteria, mycobacteria, and fungi; induced sputum for Pneumocystis jirovecii pneumonia; serum and blood tests for circulating Cytomegalovirus and Aspergillus; blood cultures; specific PCR tests for herpes viruses on blood and respiratory samples; and urine tests for bacterial antigens. Bronchoscopy and bronchoalveolar lavage (BAL) were performed when deemed appropriate by the attending physician. BAL fluid was collected as previously described19 and was used for bacterial, mycobacterial, and fungal cultures; RV antigen detection by immunofluorescence; and cytological examination. Echocardiography, chest computed tomography, and thoracocentesis were also performed when deemed appropriate by the attending physician.
Clinically documented infection was defined as a strong clinical and radiographic suspicion of pneumonia without microbiological documentation but with either septic shock or complete resolution after antibacterial treatment.5
Immunofluorescence (Argene, Verniolle, France) was performed routinely to test nasopharyngeal aspirates (NPA) and BAL fluid for influenza A and B viruses; respiratory syncytial virus (RSV); parainfluenza viruses (PIV) 1, 2, and 3; and adenoviruses. Human metapneumovirus (hMPV) was sought starting in October 2007.
All respiratory specimens were also investigated for RVs using an MMA based on the Multiplex Ligation-dependent Probe-Amplification (MLPA) technology (RespiFinder19®, Pathofinder, Maastricht, The Netherlands) that allows the detection and differentiation of 14 respiratory viruses, including influenza viruses A and B; PIV-1 to PIV-4; RSV A and B; rhinovirus; human coronaviruses 229E, OC43 and NL63; hMPV; and adenovirus.20 Additionally, this MMA detects influenza A H5N1 and four bacteria (Chlamydophila pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila, and Bordetella pertussis). Samples were stored at −80°c. Before extraction, 5 μl of an internal amplification control which contained an encephalomyocarditis virus RNA transcript was added into the sample. Nucleic acids were purified from 400 μl of samples with the EasyMag system (Biom » rieux, Marcy l’Etoile, France) and eluted in 100 μl of elution buffer, of which 10 were used for amplification. The three steps of pre-amplification, hybridization and ligation-PCR were performed on a C1000 themocycler (Bio-Rad, Marnes la Coquette, France). The amplified MLPA products were analyzed on an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA). Fragment sizing analysis was performed with the GeneMarker software (SoftGenetics, LLC, State College, PA).
Two clinicians reviewed the medical charts of all study patients to determine the etiologies of ARF. This was made on the basis of clinical, radiographic, microbiological, and histological findings, according to predefined criteria.1, 6, 21 The reviewers also aimed at evaluating the clinical significance of RV detection by MMA in these patients. In the absence of validated criteria, this was mainly based on the presence or absence of a definite etiology of ARF, and on the possible interplay between viral infection and this etiology.
Statistics
Quantitative parameters are reported as median and interquartile range (IQR, 25th −75th percentiles) and qualitative parameters as number and percentage. Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate. Continuous variables were compared using the Mann–Whitney U test or the Wilcoxon test, as appropriate. Associations between patient characteristics and positive MMA results were assessed using a logistic regression model. Multivariable analysis was performed using stepwise forward selection to introduce variables yielding P values smaller than 0.20 by univariate analysis. We also introduced variables that seemed clinically relevant. Then, the absence of a significant increase in the likelihood value after omission of each of the remaining variables was checked. Odds ratios (ORs) and their 95% confidence intervals (95%CIs) were computed. P values less than 0.05 were considered significant. Statistical analyses were performed using Statview 5.0 (SAS Institute, Cary, NC).
Results
We included 100 immunocompromised patients admitted to our ICU with ARF during the study period. Their main characteristics are listed in Table 1. Median symptom duration at ICU admission was 31, 2, 3, 4, 5, 6 days. The admission leukocyte count was 7.7 (3.4–14.9) 109 L−1, and 6 (6%) patients had neutropenia at admission. At admission, PaO2 was 11.3 (8.7–14.6) kPa with a mean oxygen rate of 62, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 L·min−1, PaCO2 was 4.88 (4.00–5.9) kPa, and pH was 7.42 (7.37–7.46). The use of life-supporting interventions and outcomes over time are reported in Table 1.
The MMA was performed on NPA in 45 (45%) patients and BAL fluid in 55 (55%). Immunofluorescence was positive in 8 (8%) patients and the MMA in 47 (47%) patients (P = 0.006) (Table 2). After excluding rhinoviruses and coronaviruses, the difference remained highly significant (31% versus 8%, P < 0.0001). Co-infection with two viruses was detected in 5 patients using the MMA; the combinations were influenza A and rhinovirus, influenza A and hMPV, influenza A and adenovirus, influenza B and adenovirus, and PIV 2 and coronavirus OC43.
Table 1 compares patients with and without a positive MMA. At ICU admission, there were no significant differences in symptom duration (4 [2–7] days vs. 3 [1–6] days, respectively; P = 0.23), PaO2 (11 [8.8–15.4] kPa vs. 11.4 [8.5–14], respectively; P = 0.47), PaCO2 (5.2 [4.0–5.9] mmHg vs. 4.7 [4.0–5.9], respectively; P = 0.31), pH (7.41 [7.36–7.45] vs. 7.44 [7.37–7.46], respectively; P = 0.36), or leukocyte count (8.0 [3.1–14.0] 109 L−1 vs. 7.2 [3.6–15.6]109 L−1, respectively; P = 0.67). By univariate analysis, a positive MMA was associated with being a solid organ transplant recipient (P = 0.01), receiving immunosuppressants in the past month (P = 0.05), and being tested on NPA (60% vs. 32%, P = 0.01). No variable was significantly associated with a positive MMA by multivariate analysis.
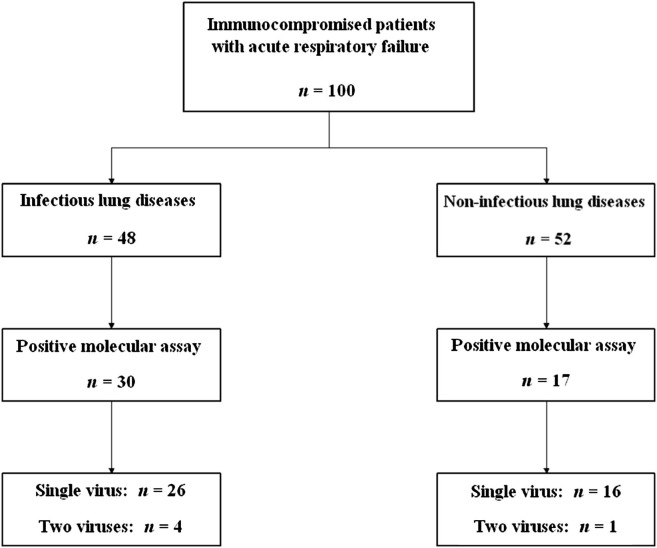
Study patients were classified into diagnostic categories based on a medical chart review (Fig. 1 ). The MMA was positive in 30 of the 48 patients with lung infections and 17 of the 52 patients with non-infectious lung diseases. Results of microbial investigations are given in Table 3. Co-infections with an RV and bacteria or fungi were found in 11 patients with infectious lung disease. These co-infections are described in Table 4 . All patients were considered as having bacterial or fungal lung infection. A patient with non-infectious lung disease had a respiratory sample positive with coagulase-negative Staphylococci and coronavirus NL63. These pathogens were not considered significant. Also, Table 3 compares the MMA-positive and MMA-negative patients in the subgroups with and without lung infection. In the subgroup of patients with non-infectious lung diseases, 12 patients had no diagnosis found at the end of the etiologic investigations. Multiplex molecular assay was positive in 6 (50%) of these patients.
Figure 1.
Multiplex molecular assay results according to the cause of acute respiratory failure.
Table 4.
Co-infection with a respiratory virus and bacteria or fungi in patients with and without infectious lung disease.
| Bacteria or fungus | RV detected by MMA | Immunofluorescence | |
|---|---|---|---|
| Patients with infectious lung disease | |||
| Patient 1 | Escherichia coli | PIV-3 | Negative |
| Patient 2 | Staphylococcus aureus | PIV-2 and coronavirus OC43 | Negative |
| Patient 3 | Staphylococcus aureus | Adenovirus | Negative |
| Patient 4 | Pseudomonas aeruginosa | Influenza B | Negative |
| Patient 5 | Streptococcus pneumoniae | Adenovirus | Negative |
| Patient 6 | Pneumocystis jirovecii | Rhinovirus | Negative |
| Patient 7 | Aspergillus sp. | RSV | Positive |
| Patient 8 | Pneumocystis jirovecii | Rhinovirus | Negative |
| Patient 9 | Aspergillus sp. | Rhinovirus | Negative |
| Patient 10 | Pneumocystis jirovecii | Rhinovirus | Negative |
| Patient 11 | Pneumocystis jirovecii | Rhinovirus | Negative |
| Patient with non-infectious lung disease | |||
| Patient 1 | Coagulase-negative Staphylococci | Coronavirus NL63 | Negative |
MMA, multiplex molecular assay; PIV, parainfluenza virus; RSV, respiratory syncytial virus.
Discussion
Among immunocompromised patients admitted to the ICU with ARF, about half had an RV detected by MMA, which was more than 5-fold the rate of RV detection by immunofluorescence. The MMA still had a significantly higher sensitivity when we considered only the viruses included in the immunofluorescence panel. All viruses detected by immunofluorescence were also detected by the MMA.
To our knowledge, this is the first MMA study in immunocompromised patients with ARF. About half these patients had at least one RV detected by the MMA. This high sensitivity is consistent with the results of previous studies in HSCT recipients with acute respiratory illnesses.15, 16 Not surprisingly, rhinoviruses were the RVs most commonly detected by the MMA in our population.15, 16, 22 Rhinoviruses and coronaviruses are not detected by immunofluorescence, a fact that may explain the higher sensitivity of the MMA. However, the MMA remained significantly more sensitive than immunofluorescence when we considered only the RVs detected by both methods.
Whether the higher sensitivity of RV by molecular screening improves the etiologic diagnosis of ARF remains uncertain. The significance of a positive MMA in immunocompromised patients with ARF cannot be determined from our data. In our study cohort, the need for life-supporting interventions and the mortality rate were both low in MMA-positive patients, but neither was significantly different from that in MMA-negative patients. The clinical and radiographic presentations were also similar. A positive MMA with negative results of other tests for viruses has been associated with lower viral loads and fewer respiratory symptoms compared to concomitant detection by MMA and other tests. Thus, PCR-based methods may help to detect asymptomatic or mildly symptomatic stages of RV infections.14 Studies have established that RVs including PIV, hMPV, and rhinoviruses are sometimes detected by MMA in respiratory samples from symptom-free HSCT, indicating that asymptomatic RV shedding can occur.16, 17, 23 RV shedding lasts longer in immunocompromised patients than in immunocompetent patients,17, 24, 25, 26 and a positive MMA may merely indicate a low level of shedding of limited clinical significance. Therefore, in some of our patients, a positive MMA may have indicated asymptomatic shedding unrelated to the cause of the ARF. Finally, MMAs detect only the nucleic acids of RVs, so that a positive MMA can be related to sample contamination.
In the subgroup of patients without documented lung infections, a positive MMA was associated with significantly less use of mechanical ventilation, a significantly shorter duration of hemodynamic failure, and nonsignificantly lower values for use of renal replacement therapy, time in the ICU, and ICU mortality. Thus, in immunocompromised patients with ARF, the MMA may identify patients with probable viral pulmonary involvement, a diagnosis associated with better outcomes than those assumed to be present when all tests are inconclusive. Indeed, negative tests for the cause of ARF constitute a major diagnostic criterion for severe non-infectious conditions such as drug-related pulmonary toxicity or pulmonary infiltration by the malignancy. Consequently, MMA may deserve to be added to the list of investigations performed routinely to detect the cause of ARF. Isn't so, every effort should be done to make the difference between a positive test and a diagnostic test. In contrast, in the subgroup of patients with lung infections, there were no significant differences between MMA-positive and MMA-negative patients. When there is a known bacterial or fungal lung infection, identifying an RV probably has no major impact on the management strategy or patient outcome. An RV may act merely as a risk factor for other infections or play a role in generating ARF, either alone or in conjunction with a bacterial or fungal infection.
Finally, the higher sensitivity of MMA compared to immunofluorescence seems to have only limited clinical consequences. The high frequency of RVs detection does not appear to have significant impact on patients' presentation and outcomes. This may be particularly true in our cohort of critically ill patients. To gain insight into the clinical significance of a positive MMA, routinely performing the MMA on both BAL fluid and NPA and/or obtaining lung biopsies might have been of interest. Histology can show a cytopathic effect but lacks sensitivity. Positive results are difficult to interpret, and no criteria are available for determining the causal role for the RV in the lung disease. High viral loads are more often accompanied with clinical disease, but low viral loads do not exclude clinical disease due to the virus. Moreover, the pathogenesis of viral infection depends not only on intrinsic viral pathogenicity, but also on genetic host factors such as those involved in the immune response. For rhinoviruses in particular, the clinical manifestations may reflect the immune response to the infection rather than the viral cytopathic effect.27
Conclusion
In our prospective cohort of immunocompromised patients admitted to the ICU with ARF, an MMA was far more sensitive than immunofluorescence for RV detection. The clinical characteristics and outcomes were not significantly different between MMA-positive and MMA-negative patients. However, the MMA can suggest alternative diagnoses to non-infectious lung diseases. Studies evaluating how this diagnostic strategy translates into improved diagnostic efficacy with further increased survival are warranted.
Financial support
This study was supported by a grant from the Assistance-Publique Hôpitaux de Paris [AOM 04139], a nonprofit institution.
Authors' contributions to the study
Study mentoring:Benoit Schlemmer and Élie Azoulay.
Study design, data collection and analysis:Élie Azoulay, Jérôme Legoff and David Schnell.
Preparation and critical reviewing of the manuscript:all authors.
Conflict of interest statement
No potential conflicts of interest occurred for any of the authors.
Acknowledgments
This study was supported by a grant from the Assistance-Publique Hôpitaux de Paris (AOM 04139), a nonprofit institution. Assistance-Publique Hôpitaux de Paris was not involved in the study design, data collection or analysis, writing of the manuscript, or decision to submit the manuscript for publication.
References
- 1.Azoulay E., Thiery G., Chevret S., Moreau D., Darmon M., Bergeron A. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine (Baltimore) 2004 Nov;83(6):360–370. doi: 10.1097/01.md.0000145370.63676.fb. [DOI] [PubMed] [Google Scholar]
- 2.Soares M., Salluh J.I., Spector N., Rocco J.R. Characteristics and outcomes of cancer patients requiring mechanical ventilatory support for >24 hrs. Crit Care Med. 2005 Mar;33(3):520–526. doi: 10.1097/01.ccm.0000155783.46747.04. [DOI] [PubMed] [Google Scholar]
- 3.Hilbert G., Gruson D., Vargas F., Valentino R., Gbikpi-Benissan G., Dupon M. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001 Feb 15;344(7):481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E., Alberti C., Bornstain C., Leleu G., Moreau D., Recher C. Improved survival in cancer patients requiring mechanical ventilatory support: impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001 Mar;29(3):519–525. doi: 10.1097/00003246-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E., Mokart D., Rabbat A., Pene F., Kouatchet A., Bruneel F. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure: prospective multicenter data. Crit Care Med. 2008 Jan;36(1):100–107. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 6.Rano A., Agusti C., Jimenez P., Angrill J., Benito N., Danes C. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001 May;56(5):379–387. doi: 10.1136/thorax.56.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Medrano F., Aguado J.M., Lizasoain M., Folgueira D., Juan R.S., Diaz-Pedroche C. Clinical implications of respiratory virus infections in solid organ transplant recipients: a prospective study. Transplantation. 2007 Oct 15;84(7):851–856. doi: 10.1097/01.tp.0000282788.70383.8b. [DOI] [PubMed] [Google Scholar]
- 8.Martino R., Ramila E., Rabella N., Munoz J.M., Peyret M., Portos J.M. Respiratory virus infections in adults with hematologic malignancies: a prospective study. Clin Infect Dis. 2003 Jan 1;36(1):1–8. doi: 10.1086/344899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnell D., Mayaux J., de Bazelaire C., Legoff J., Feuillet S., Scieux C. Risk factors for pneumonia in immunocompromised patients with influenza. Respir Med. 2010 Feb 22 doi: 10.1016/j.rmed.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Chemaly R.F., Ghosh S., Bodey G.P., Rohatgi N., Safdar A., Keating M.J. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006 Sep;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 11.Couch R.B., Englund J.A., Whimbey E. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997 Mar 17;102(3A):2–9. doi: 10.1016/S0002-9343(97)00003-X. discussion 25–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt A.C., Alexander R., Hibbert J., Deed N., Barr I.G. Performance of six influenza rapid tests in detecting human influenza in clinical specimens. J Clin Virol. 2007 Jun;39(2):132–135. doi: 10.1016/j.jcv.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Kuypers J., Wright N., Ferrenberg J., Huang M.L., Cent A., Corey L. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006 Jul;44(7):2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuypers J., Campbell A.P., Cent A., Corey L., Boeckh M. Comparison of conventional and molecular detection of respiratory viruses in hematopoietic cell transplant recipients. Transpl Infect Dis. 2009 Aug;11(4):298–303. doi: 10.1111/j.1399-3062.2009.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Elden L.J., van Kraaij M.G., Nijhuis M., Hendriksen K.A., Dekker A.W., Rozenberg-Arska M. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin Infect Dis. 2002 Jan 15;34(2):177–183. doi: 10.1086/338238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Kraaij M.G., van Elden L.J., van Loon A.M., Hendriksen K.A., Laterveer L., Dekker A.W. Frequent detection of respiratory viruses in adult recipients of stem cell transplants with the use of real-time polymerase chain reaction, compared with viral culture. Clin Infect Dis. 2005 Mar 1;40(5):662–669. doi: 10.1086/427801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peck A.J., Englund J.A., Kuypers J., Guthrie K.A., Corey L., Morrow R. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007 Sep 1;110(5):1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993 Dec 22-29;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay E., de Miranda S., Bele N., Schlemmer B. Diagnostic strategy for acute respiratory failure in patients with haematological malignancy. Rev Mal Respir. 2008 Apr;25(4):433–449. doi: 10.1016/s0761-8425(08)71584-5. [DOI] [PubMed] [Google Scholar]
- 20.Reijans M., Dingemans G., Klaassen C.H., Meis J.F., Keijdener J., Mulders B. RespiFinder: a new multiparameter test to differentially identify fifteen respiratory viruses. J Clin Microbiol. 2008 Apr;46(4):1232–1240. doi: 10.1128/JCM.02294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afessa B., Tefferi A., Litzow M.R., Peters S.G. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002 Nov 15;166(10):1364–1368. doi: 10.1164/rccm.200208-792OC. [DOI] [PubMed] [Google Scholar]
- 22.Bowden R.A. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997 Mar 17;102(3A):27–30. doi: 10.1016/s0002-9343(97)00007-7. discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 23.Debiaggi M., Canducci F., Sampaolo M., Marinozzi M.C., Parea M., Terulla C. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006 Aug 15;194(4):474–478. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 24.Weinstock D.M., Gubareva L.V., Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003 Feb 27;348(9):867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 25.Boivin G., Goyette N., Bernatchez H. Prolonged excretion of amantadine-resistant influenza a virus quasi species after cessation of antiviral therapy in an immunocompromised patient. Clin Infect Dis. 2002 Mar 1;34(5):E23–E25. doi: 10.1086/338870. [DOI] [PubMed] [Google Scholar]
- 26.Nichols W.G., Guthrie K.A., Corey L., Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004 Nov 1;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 27.Gern J.E., Vrtis R., Grindle K.A., Swenson C., Busse W.W. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000 Dec;162(6):2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]