Abstract
The effect of vaccinating pregnant cows with an inactivated vaccine against Mannheimia haemolytica, BRSV and PI3V infections on selected immune responses in their offspring was examined. Blood samples were collected weekly for 12 weeks from six newborn calves from each of vaccinated (experimental) and unvaccinated (control) dams. Specific antibodies to M. haemolytica, BRSV and PI3V and mean values of IgA, IgG concentrations were significantly higher in the experimental calves compared with the controls. However, specific antibody titres to adenovirus type 3, BHV1 and BVDV in the experimental calves had constant levels while the control group levels changed. The IgM, Hp and SAA concentrations generally increased until week 8 in the experimental group, but the control group titres became higher after week 9. This study demonstrates that specific immunisation of cows pre-partum significantly stimulated parameters associated with immunity and it also controlled the acute phase response intensity in their offspring. Therefore the vaccination of dams may provide additional antibody protection against infection to their offspring.
Keywords: Mannheimia haemolytica, BRSV, PI3V, Vaccine, Immune response
1. Introduction
The development of the innate immune system is essential for new-born animals to survive, especially when exposed to infectious diseases that are responsible for high morbidity and mortality. During the first few months of life new-born calves have a weakened immunity as the granulocyte function and complement activity are low (Cervenak, Kacskovics, 2009, Cortese, 2008); and the calves also lack specific immunity (Boysen et al, 2006, Stefaniak et al, 2012). Protection obtained from colostrum falls as the immunoglobulins decline usually from the seventh day of life. This is at a time when their specific antibodies are at a low concentration; which then develops as the calves grow (Stefaniak et al., 2011). During this period of low immunity the animals are more susceptible to infections including bovine respiratory disease (BRD). BRD is a complex and multifactorial disease caused by bacteria and viruses and is one of the most important diseases of cattle. Estimated losses to the cattle industry from BRD is more than US$3 billion every year (Griffin, 1997). The main causative bacteria are: Mycoplasma bovis, and Mycoplasma dispar for which there are currently no commercial vaccines; and the Pasteurellaceae family of which the most pathogenic is Mannheimia haemolytica (Singh et al., 2011). Viruses including PI3V and BRSV have an important role in BRD, not only in the pathogenesis of the disease but also by suppressing the host immunity (Cusack et al., 2003). Calves are usually vaccinated during the first weeks of life. However, calf immunisation may be adversely affected by interference from maternal antibodies or unfavourable environmental conditions. Therefore, the effective vaccination of pregnant cows and the subsequent colostrum intake by their offspring can enhance the immune response of new-born calves.
The aim of the study was to investigate the level of passive immunity acquired by calves that have been fed colostrum derived only from their own dam which was vaccinated pre-partum for M. haemolytica, PI3V and BRSV, or from their own unvaccinated dam in the control group.
2. Material and methods
2.1. Experimental procedure
To investigate the effect of stimulating the calf's immune system by vaccinating their dams, pregnant cows were vaccinated with BRSV-PI3V-M. haemolytica antigens. The immune response in the calves was analysed and multiple parameters measured as described later.
2.2. Cattle
Twelve pregnant Holstein Friesians cows were sourced from a commercial 2000 head dairy farm. Two groups of six cows were housed separately. Experimental procedures and animal management protocols were carried out in accordance with the requirements of the Local Ethics Committee on Animal Experimentation.
2.3. Vaccination of pregnant cows
One group of six cows were vaccinated with 5 ml of inactivated vaccine composed of BRSV-PI3V-M. haemolytica antigens (Bovilis®Bovipast RSP, MSD Animal Health, The Netherlands). These subcutaneous vaccinations were in the lateral neck region and were done twice, at 8 and 4 weeks, before the expected delivery dates. The same experimental design was carried out for the six remaining cows administrating PBS.
2.4. The calves
Directly after parturition the new-born calves were separated from their dams and kept in the separate groups from vaccinated–‘experimental’ and unvaccinated dams. The calves were kept according to the standard breeding regulations and fed colostrum derived from their own dams by oral pathway (in a dose of 2 L per calf three times a day) for three consecutive days after delivery. The total level of colostrum immunoglobulins was determined using a colostrometer for the measurement of specific gravity in bovine colostrum. After that the calves were kept in individual pens and fed with raw milk without the presence of any contaminated infectious agents for 1 month and then fed milk replacer. Rectal temperatures, general condition and the presence of respiratory signs were recorded daily. Blood samples for laboratory analysis were collected from vena jugularis externa of calves at weekly intervals for 12 weeks.
2.5. The analyses of immune parameters
Mannheimia haemolytica specific antibody levels were determined using an in-house ELISA as described by Makoschey et al. (2012).
A commercial respiratory ELISA kit (Bio-X Diagnostics, Belgium) was used to obtain the antibody levels to five bovine viruses (BRSV, PI3V, adenovirus type 3, BHV-1 and BVDV). The optical densities in the microwells were read at 450 nm. The signal read for each sample well was divided by the corresponding positive control serum signal and multiplied by 100 to express a result as a percentage (Val). The sample was considered as positive for BRSV by Val > 10.51, for PI3V by Val > 9.56, for adenovirus type 3 by Val > 11.86, for BHV-1 by Val > 10.08 and for BVDV by Val > 10.27, respectively. The values expressed as a percentage (Val) correspond with a degree of positivity of each serum described in the table in the quality control procedure (Quality control, Bio-X Diagnostics, Belgium). The ranges of positivity degrees for each viral agent are described in the figure legends (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5 respectively).
Fig. 1.
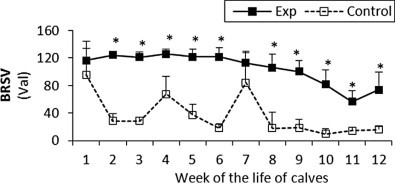
The percentage (Val) of monoclonal antibodies specific to BRSV in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
Fig. 2.
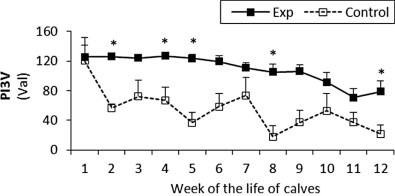
The percentage (Val) of monoclonal antibodies specific to PI3V in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
Fig. 3.
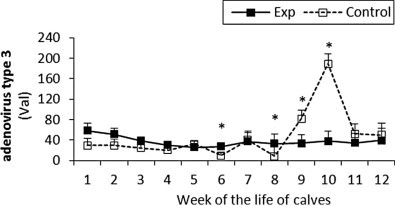
The percentage (Val) of monoclonal antibodies specific to adenovirus type 3 in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
Fig. 4.
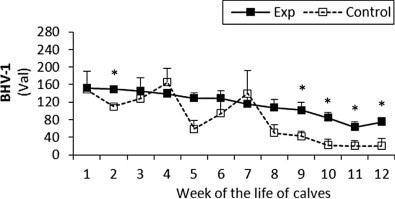
The percentage (Val) of monoclonal antibodies specific to BHV-1 in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
Fig. 5.
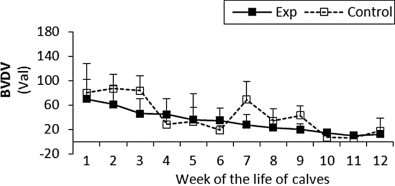
The percentage (Val) of monoclonal antibodies specific to BVDV in sera of experimental and control calves from the 1st to 12th week of the animal life. Exp, experimental calves; Control, control calves.
Commercial kits were used following the manufacturer's instructions to analyse gamma globulin concentration (Bio-X Diagnostics, Belgium), Ig classes (Bethyl Laboratories, Inc.), haptoglobulin (Hp) and serum amyloid A (SAA) which was measured using two different commercial ELISA kits (Tridelta Development Limited, Ireland). Standard curves for each parameter were determined and optical densities were determined at OD450 unless otherwise stated. Dilution series of standard curves were constructed using different standards/calibrators provided in the kits at 234.8 , 156.5, 104.4, 69.6, 46.4, 30.9, 20.6 and 13.7 µg/ml for gamma globulin (the bovine blood serum, Bio-X Diagnostics, Belgium); 500, 250, 125, 62.5, 31.25, 15.625, 7.8 and 0 ng/ml (blank) for IgG (the bovine reference serum, Bethyl Laboratories, Inc.); 1000, 500, 250, 125, 62.5, 31.25, 15.625 and 0 ng/ml (blank) for IgA and IgM (two different bovine reference sera, Bethyl Laboratories, Inc.). 2.5, 1.25, 0.625, 0.312 and 0 mg/ml for Hp (the Hp calibrator, Tridelta Development Limited, Ireland) measured at OD630; and 300, 150, 75, 37.5, 18.8 and 0 ng/ml for SAA (the SAA calibrator, Tridelta Development Limited, Ireland) which were read at OD450 and OD630 as a reference. If the samples were diluted the result was multiplied by the dilution factor.
For all of the parameters examined the optical densities in the microwells were read using an automated plate reader (Elx800 Microplate Reader, BioTek Instruments, Inc., USA). The data were collected using the KC Junior programme manufactured by BioTek Instruments, Inc. (USA). From previous studies the described parameters have a normal distribution (NN).
2.6. Statistical analyses
The results are presented as arithmetic means. The differences between the mean values recorded in the experimental and control groups at the same time point were analysed using non-paired Student's t-test with a statistically significant level of p < 0.05.
3. Results
Normal rectal temperatures (38.0–39.5 °C) and no respiratory signs were observed in any of the calves throughout the study. The ‘experimental’ calves from the vaccinated dams had higher body weight gains, lower morbidity index and improved feed conversion ratios when compared to the control group, but no clinical differences were observed.
The colostrum of unvaccinated cows specific gravity ranged from 1.045 to 1.050 which correlates to an Ig concentration of 47.2–62.5 mg/ml. However, the specific gravity in the colostrum derived from the vaccinated dams was more than 1.050 (>62.5 mg/ml of Ig).
In the experimental group the M. haemolytica specific antibody levels were >12.6 (2LOG) for the first 3 weeks compared to 10.2 (2LOG) in the control group. These values declined slightly but the experimental group titres remained higher than the control groups throughout the experiment (Table 1 ).
Table 1.
The titres of anti-Mannheimia haemolytica specific antibodies in experimental and control calves from the 1st to 12th week of the animal life.
| Weeks of animal life | Animals | |
|---|---|---|
| Exp | Control | |
| 1 | >12.60 ± 0.00* | 10.12 ± 0.52 |
| 2 | >12.60 ± 0.00* | 11.13 ± 0.79 |
| 3 | >12.60 ± 0.00* | 9.40 ± 0.91 |
| 4 | >12.50 ± 0.10* | 9.78 ± 0.46 |
| 5 | >12.15 ± 0.59* | 9.40 ± 0.36 |
| 6 | 12.28 ± 0.28* | 9.12 ± 0.41 |
| 7 | >10.83 ± 0.99* | 8.57 ± 1.23 |
| 8 | >11.90 ± 0.48* | 9.37 ± 1.29 |
| 9 | 11.47 ± 0.67* | 8.40 ± 0.68 |
| 10 | 11.03 ± 0.33* | 9.08 ± 0.65 |
| 11 | 10.40 ± 0.78* | 8.70 ± 0.36 |
| 12 | 10.30 ± 0.55* | 8.60 ± 0.36 |
*Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves. The results are expressed as 2LOG (the mean values ± standard deviations).
The BRSV and PI3V antibody titres were significantly higher in the experimental calves throughout the study (Fig. 1, Fig. 2 respectively). For adenovirus type 3 in the experimental calves titres were generally higher than control values until the eighth week, however after that the control levels rapidly increased and remained higher than the experimental values until the end of the study (Fig. 3). With the exception of the fourth and seventh week the antibody titres to BHV1 in the experimental calves was higher than the control values throughout the study (Fig. 4). With the exception of the second, third, seventh and ninth week when antibodies titres to BVDV were higher in the control group, similar titres were observed for both groups throughout the study (Fig. 5).
The gamma globulin concentration was stable and had comparable values in both groups until week 7 when the experimental group showed a steady increase in levels, however by week 9 in the control group the gamma globulin concentration rapidly increased and remained higher than the experimental groups until the end of the study (Fig. 6 ).
Fig. 6.
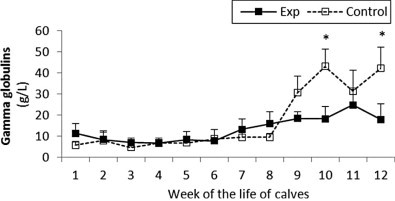
Gamma globulin concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. Exp, experimental calves; Control, control calves.
The IgA levels in the experimental group were very high at week 1 compared to the control group but dropped by week 2. However they were slightly higher throughout the study when compared to the control, with the exception of week 9. Statistically significant differences between the two groups were observed at the first, sixth and seventh week (Fig. 7 ).
Fig. 7.
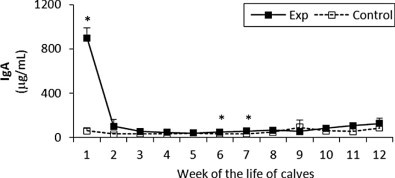
IgA concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
The IgM levels in the experimental group were very high at week 1 and then decreased but remained higher than the control group until week 8 with the exception of week 5. From the ninth week until the end of the study IgM levels in the control group were higher than experimental values. Statistically significant differences between the two groups were observed at the 1st, 2nd, 11th and 12th weeks (Fig. 8 ).
Fig. 8.
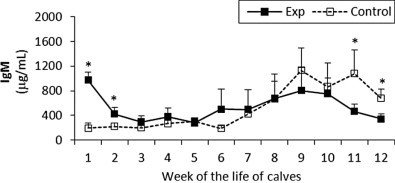
IgM concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
The levels of IgG in the control calves remained stable throughout the study. In contrast, the experimental group had very low values at week 1 and then were consistently higher when compared to the control group until the end of the study at week 12. Statistically significant differences between the groups were observed throughout the study (Fig. 9 ).
Fig. 9.
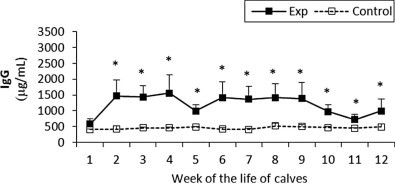
IgG concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
In the experimental animals Hp concentration increased in 10-week-old calves but returned to the background level 1 week later (Fig. 10 ). However, in the control animals an increase in Hp concentrations occurred at week 9 and continued to a maximum value of 1.14 mg/ml by the end of the experiment in week 12 (almost six times higher than seen in the experimental calves).
Fig. 10.
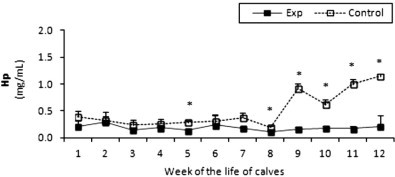
Haptoglobin (Hp) concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
The SAA concentration was significantly higher in the experimental calves compared to the control values at different time points until the ninth week. Then the concentration increased in the control group while the experimental group declined giving a statistically significant differences until week 12 (Fig. 11 ).
Fig. 11.
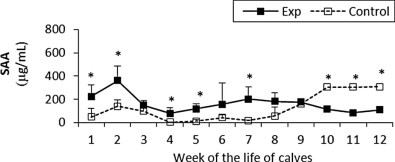
Serum amyloid A (SAA) concentration in sera of experimental and control calves from the 1st to 12th week of the animal life. *Significant differences (p < 0.05) within the two groups of calves. Exp, experimental calves; Control, control calves.
4. Discussion
Attempts to protect calves from certain infections by feeding them colostrum and milk from cows vaccinated pre-partum have been successful in many cases (Makoschey et al, 2012, Rabinovitz et al, 2012). This model for protecting calves was used in preventing diarrhoea caused by both bacterial and viral pathogens. Pre-partum vaccination of cows with different virulence factors of enterohaemorrhagic serotype of Escherichia coli effectively stimulated the production of specific antibodies in the colostrum and milk fed to the offspring. Therefore, the passive protection of calves may prevent the early colonisation of the bacteria and then the development of diarrhoea in the calves (Rabinovitz et al., 2012). Similar results were shown in another study, where a combined vaccine against rotavirus, coronavirus and E. coli was used to immunise pregnant cows. In these studies high titres of specific antibodies were measured in the colostrum and milk of dams to three components of the applied vaccine (Crouch et al., 2001).
Makoschey et al. (2012) have shown the effect of colostral antibodies from cow's vaccinated pre-partum with Bovilis®Bovipast RSP on the passive immunity of their offspring. Pre-vaccination of the cows partially protected the calves against the infection with M. haemolytica which was evident from the higher level of M. haemolytica specific antibodies and lower calf mortality when compared to the control group.
Bovilis®Bovipast RSP vaccine has three components based on inactivated strains of serotype 1 and biotype A of M. haemolytica and two viruses, PI3V and BRSV. M. haemolytica is considered to be a primary bacterial pathogen involved in BRD (Reddy et al., 2012). The high mortality of cattle following infection with M. haemolytica results from numerous virulence factors of the bacteria responsible for cytolysis and apoptosis of leukocytes, endotoxin activity, colonisation, modulation of leukocyte activities like phagocytosis, avoiding the immunological system of the host by the resistance to complement lysis or masking of cell surface and others (Lo, 2001). Some of the virulence factors of M. haemolytica have strong immunogenic properties, like leukotoxin (Bednarek et al., 2009) or outer membrane proteins (OMPs) (Ayalew et al., 2011). Bovilis®Bovipast RSP vaccine contains the so-called iron regulated outer membrane proteins (IROMPs) of M. haemolytica. The phenomenon of IROMPs relies on facilitating the survival of the bacteria in a state of iron deficiency (sideropenia); this is also used by the host as one of its natural defences against infection. Therefore, the ability of the bacteria to survive in adverse conditions may affect its growth and the effective stimulation of the humoral immune response.
In this study, in the experimental calves a marked increase in M. haemolytica specific antibody was observed, as was the stimulation of specific antibodies to BRSV and PI3V. These viruses are also important in BRD aetiology due to their immunosuppressive properties (Cusack et al., 2003). High level of specific antibodies to M. haemolytica and the vaccine viruses, observed in this study in experimental calves from the first weeks of their life, pointed to the transfer of passive immunity derived from the vaccinated dams. However, specific antibody titres to adenovirus type 3, BHV1 and BVDV in the experimental calves were constant while the titres in the control groups changed throughout the course of the study. Following the colostrum administration derived from the vaccinated dams a more stable innate immunity status was observed in the calves fed with the natural secretion than in the controls. This passive intake of specific antibodies with colostrum in the experimental calves also enhanced their immune response against other infectious viral agents. In contrast this response in the control animals was expressed less which may account for the increase in the adenovirus type 3 titre observed in the control calves. One explanation for this response in the control group is the possible inverse correlation between colostrum administration and protection against adenovirus type 3 (Corbeil et al, 1984, Valarcher, Hägglund, 2006).
A study performed by Furman-Fratczak et al. (2011) on heifer calves showed positive correlation between the high serum level of gamma globulins and the health status of the animals. In this study an unexpected increase in gamma globulin concentration in the control group could be as a result of an observed increase in IgM which coincided with infection and the humoral immune response. The gamma globulins also increased in the experimental calves which also correlated with raised IgG concentrations, resulting from the passive immunity transfer from the pre-partum vaccinated dams. The transfer of IgG via colostrum is very important to calves especially due to the lack of the maternal immunoglobulin ability to permeate through the placenta (Barrington and Parish, 2001). The increased IgA levels in experimental calves observed here could be as a result of both the passive colostrum transfer and the mucosal immunity stimulation within the mucosa-associated lymphoid tissue (MALT) which effectively prevents pathogen colonisation and its proliferation (Underdown and Schiff, 1986).
Acute phase proteins (APPs) are known as biomarkers of diseases in animal health and veterinary medicine. APPs are synthesised in response to infection or inflammation and their production is mediated by proinflammatory cytokines. In cattle the most indicative and useful APPs are Hp and SAA (Eckersall and Bell, 2010). Changes in concentrations of these proteins were found in the course of infections with different microorganisms, such as M. haemolytica, Pasteurella multocida, BRSV and many other bovine diseases like bovine viral diarrhoea, foot and mouth disease, mastitis, respiratory disease, metritis and hepatic lipidosis (Petersen et al., 2004). Our previous studies showed an increase in the concentration of both APPs in response to experimental infections in calves with a pathogenic strain of Mycoplasma bovis (Dudek et al., 2010) and from natural mycoplasmal infections (Dudek et al., 2011). In this study, the ninth week of the calf's life was critical for the control animals, as this was when the Hp concentration increased and the most of the changes in the immunological parameters analysed here were observed. This suggests that the pre-partum specific immunisation of the cows stabilised the acute phase response in their offspring, which was most visible during the infections. It was also observed in this study which may be as a result of an adenovirus type 3 infection in the control calves, which was not confirmed. The Hp changes in these animals correlated with an increase in the adenovirus type 3 titre.
Pre-partum immunisation of the cows with the specific vaccine against bovine respiratory infections may effectively stimulate the natural immune response in their offspring. It can enhance the active vaccination of calves against these pathogens during early stages of life, when the immune response is developing, and stress, infections and others factors make effective immunisation difficult. This study has demonstrated the stimulation of the natural immune response in the offspring by the vaccination of their pregnant dams. Further studies to challenge the protective immunity of that response would be beneficial.
Acknowledgements
The authors thank Dr. Birgit Makoschey for suggestions in preparing the article and the staff in the Service Lab of MSD-Animal Health, Boxmeer for the technical assistance in determination of M. haemolytica specific antibodies.
References
- Ayalew S., Shrestha B., Montelongo M., Wilson A.E., Confer A.W. Identification and immunogenicity of Mannheimia haemolytica S1 outer membrane lipoprotein PlpF. Vaccine. 2011;29:8712–8718. doi: 10.1016/j.vaccine.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Barrington G.M., Parish S.M. Bovine neonatal immunology. The Veterinary Clinics of North America. Food Animal Practice. 2001;17:463–476. doi: 10.1016/S0749-0720(15)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek D., Urban-Chmiel R., Dudek K., Szymańska-Czerwińska M. Evaluation of peripheral blond leukocyte subpopulations by flow cytometry in calves treated with Mannheimia haemolytica leukotoxin. Bulletin of the Veterinary Institute in Pulawy. 2009;53:199–203. [Google Scholar]
- Boysen P., Olsen I., Berg I., Kulberg S., Johansen G.M., Storset A.K. Bovine CD2-/NKp46+ cells are fully functional natural killer cells with a high activation status. BMC Immunology. 2006;27:7–10. doi: 10.1186/1471-2172-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenak J., Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Veterinary Immunology and Immunopathology. 2009;128:171–177. doi: 10.1016/j.vetimm.2008.10.300. [DOI] [PubMed] [Google Scholar]
- Corbeil L.B., Watt B., Corbeil R.R., Betzen T.G., Brownson R.K., Morrill J.L. Immunoglobulin concentrations in serum and nasal secretions of calves at the onset of pneumonia. American Journal of Veterinary Research. 1984;45:773–778. [PubMed] [Google Scholar]
- Cortese V.S. Neonatal immunology. The Veterinary Clinics of North America. Food Animal Practice. 2008;25:221–227. doi: 10.1016/j.cvfa.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch C.F., Oliver S., Francis M.J. Serological, colostral and milk responses of cows vaccinated with a single dose of a combined vaccine against rotavirus, coronavirus and Escherichia coli F5 (K99) The Veterinary Record. 2001;149:105–108. doi: 10.1136/vr.149.4.105. [DOI] [PubMed] [Google Scholar]
- Cusack P.M.V., McMeniman N., Lean I.J. The medicine and epidemiology of bovine respiratory disease in feedlots. Australian Veterinary Journal. 2003;81:480–487. doi: 10.1111/j.1751-0813.2003.tb13367.x. [DOI] [PubMed] [Google Scholar]
- Dudek K., Bednarek D., Szymańska-Czerwińska M. Acute phase response in calves as a result of experimental challenge with Mycoplasma bovis. Bulletin of the Veterinary Institute in Pulawy. 2010;54:517–520. [Google Scholar]
- Dudek K., Bednarek D., Szacawa E. Evaluation of immune response in seropositive cattle for Mycoplasma bovis. Bulletin of the Veterinary Institute in Pulawy. 2011;55:631–634. [Google Scholar]
- Eckersall P.D., Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. The Veterinary Journal. 2010;185:23–27. doi: 10.1016/j.tvjl.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Furman-Fratczak K., Rzasa A., Stefaniak T. The influence of colostral immunoglobulin concentration in heifer calves’ serum on their health and growth. Journal of Dairy Science. 2011;94:5536–5543. doi: 10.3168/jds.2010-3253. [DOI] [PubMed] [Google Scholar]
- Griffin D. Economic impact associated with respiratory disease in beef cattle. The Veterinary Clinics of North America. Food Animal Practice. 1997;13:367–377. doi: 10.1016/s0749-0720(15)30302-9. [DOI] [PubMed] [Google Scholar]
- Lo R.Y.C. Genetic analysis of virulence factors of Mannheimia (Pasteurella) haemolytica A1. Veterinary Microbiology. 2001;83:23–35. doi: 10.1016/s0378-1135(01)00374-1. [DOI] [PubMed] [Google Scholar]
- Makoschey B., Ramage C., Reddick D., Rnaser S., Donachie W. Colostrum from cattle immunized with a vaccine based on iron regulated proteins of Mannheimia haemolytica confers partial protection. Vaccine. 2012;30:969–973. doi: 10.1016/j.vaccine.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen J.P., Heegaard P.M. Application of acute phase protein measurements in veterinary clinical chemistry. Veterinary Research. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- Rabinovitz B.C., Gerhardt E., Tironi-Farinati C., Acdala A., Galarza R., Vilte D.A. Vaccination of pregnant cows with EspA, EspB, γ-intimin, and Shiga toxin 2 proteins from Escherichia coli O157:H7 induces high levels of specific colostral antibodies that are transferred to newborn calves. Journal of Dairy Science. 2012;95:3318–3326. doi: 10.3168/jds.2011-5093. [DOI] [PubMed] [Google Scholar]
- Reddy J.S., Kumar R., Watt J.M., Lawrence M.L., Burgess S.C., Nanduri B. Transcriptome profile of a bovine respiratory disease pathogen: Mannheimia haemolytica PHL213. BMC Bioinformatics. 2012;13(Suppl. 15):S4. doi: 10.1186/1471-2105-13-S15-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Confer A.W., Hope J.C., Rizzi T., Wyckoff J.H., 3rd, Weng H.Y. Cytotoxicity and cytokine production by bovine alveolar macrophages challenged with wild type and leukotoxin-deficient Mannheimia haemolytica. The Veterinary Journal. 2011;188:221–227. doi: 10.1016/j.tvjl.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Stefaniak T., Chełmońska-Soyta A., Leibold W. Odporność noworodków. In: Skrzypczak W., Zabielski R., editors. Fizjologia noworodka z elementami patofizjologii. PWRiL; Warszawa: 2011. pp. 145–181. [Google Scholar]
- Stefaniak T., Chełmońska-Soyta A., Bajzert J., Jawor P., Rząsa A., Sitnik O. Rozwój układu odpornościowego u przeżuwaczy w okresie pre- i postnatalnym. Medycyna Weterynaryjna. 2012;68:534–539. [Google Scholar]
- Underdown B.J., Schiff J.M. Immunoglobulin A: Strategic defense initiative at the mucosal surface. Annual Review of Immunology. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Valarcher J.F., Hägglund S. World Buiatrics Congress; Nice, France: 2006. Viral respiratory infections in cattle. [Google Scholar]


