Abstract
Objective
To describe the clinical presentation and laboratory diagnosis of pregnant women with respiratory syncytial virus (RSV) infection.
Methods
Pregnant women in their second and third trimester were enrolled during the course of routine prenatal care visits when they were asymptomatic within the preceding two weeks (healthy controls) or when they reported symptoms of acute respiratory illness (ARI) of ≤7 days of duration (cases). Clinical outcomes were assessed at enrollment and two weeks after. Re-enrollment was allowed. Nasal-pharyngeal secretions were evaluated for respiratory pathogens by real-time reverse transcription polymerase chain reaction (PCR). Sera were tested for RSV-specific antibody responses by Western Blot, microneutralization assay, and palivizumab competitive antibody assay.
Results
During the 2015–2016 respiratory virus season, 7 of 65 (11%) pregnant women with ARI at their initial enrollment and 8 of 77 (10%) pregnant women with ARI during the study period (initial or re-enrollment) had PCR-confirmed RSV infection. Four (50%) PCR-confirmed RSV ARI cases reported symptoms of a lower respiratory tract illness (LRTI), one was hospitalized. Combining PCR and serology data, the RSV attack rate at initial enrollment was 12% (8 of 65), and 13% (10 of 77) based on ARI episodes. Among healthy controls, 28 of 88 (32%) had a Western Blot profile suggestive of a recent RSV infection either in the prior and/or current season.
Conclusion
RSV had an attack rate of 10–13% among ambulatory pregnant women receiving routine prenatal care during the respiratory virus season. The serology results of healthy controls suggest a potentially higher attack rate. Future studies should be aware of the combined diagnostic strength of PCR and serology to identify RSV infection. As maternal RSV vaccine candidates are evaluated to protect young infants, additional priority should be placed on outcomes of pregnant women.
Keywords: Respiratory syncytial virus, Acute respiratory illness, Pregnancy, Maternal infection, Maternal vaccine
1. Introduction
Infection with respiratory pathogens during pregnancy can result in mild to severe acute respiratory illness (ARI). Due to the physiologic changes that occur during pregnancy, pregnant women may experience severe outcomes when infected with a respiratory pathogen [1]. Infection with influenza during pregnancy has been associated with an increased risk of maternal mortality, preterm birth, and infants that are small for gestational age [2], [3]. To prevent these adverse maternal and fetal outcomes, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommends immunization with influenza vaccine during pregnancy [4]. The ACIP also recommends maternal immunization with the Tetanus-Diphtheria-acellular Pertussis (Tdap) vaccine [5] given that, although adult infection with pertussis is often mild, maternal antibodies transferred to the infant can prevent pertussis-related morbidity and mortality in this vulnerable age group [6].
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract illness (LRTI) among infants and young children, contributing significantly to morbidity and mortality in these populations [7], [8], [9]. There is currently a robust pipeline of RSV vaccine candidates in various preclinical and clinical phases of development. One strategy targets protection of infants less than three months of age through maternal vaccination. Maternal RSV antibodies are efficiently transferred transplacentally to the fetus during gestation, and have been associated with decreased risk of RSV infection in young infants [10], [11], [12]. For this reason, maternal immunization appears to be a promising strategy to prevent severe RSV disease in infants. However, the direct benefit to pregnant women from maternal immunization against RSV is currently unknown.
Maternal RSV infection has not been well defined, both in regards to rate of infection and consequences of infection. A case-series study was the first to describe severe RSV infection during pregnancy: two of the three cases were hospitalized and required mechanical ventilation [13]. In addition, several large prospective maternal cohort studies of influenza-like illness (ILI) evaluated during influenza vaccine trials have described maternal RSV infection incidences of 3.9/1000 person-years in Nepal, 5.3/1000 person-months in South Africa, and 0.3/1000 person-days in Mongolia [14], [15], [16]. Recently, a retrospective multi-country study of ARI-related hospitalizations among pregnant women identified 846 women who were tested for RSV during the influenza season, 21 (2.5%) had detectable RSV by real-time reverse transcription polymerase chain reaction (PCR) [17]. Of the 21 cases, 13 had a pregnancy complication, 8 were diagnosed with pneumonia, and 6 had subsequent preterm birth. We previously described the frequency and clinical impact of respiratory viruses in women seen during the second and third trimester of pregnancy at a primary obstetrical and gynecological clinic [18]. In this manuscript we expand on the clinical and diagnostic laboratory findings of pregnant women infected with RSV.
2. Materials and methods
2.1. Study design
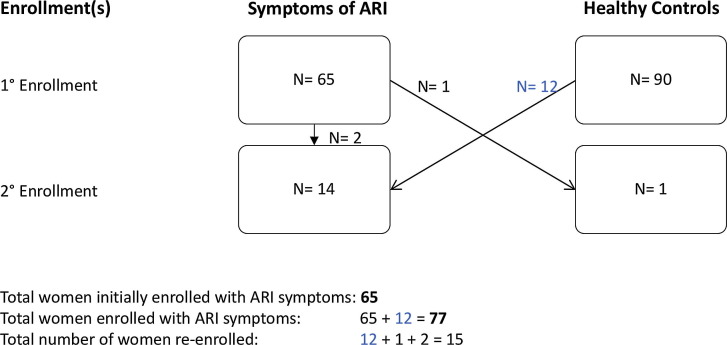
Detailed methods for patient enrollment and sample collection have been previously published [18]. In summary, pregnant women in their second or third trimester of gestation were enrolled from November 3 through May 10, during the 2015–2016 respiratory virus season, as defined by local surveillance, as they received routine prenatal care at an obstetrical/gynecological clinic in Houston, Texas, whether they were asymptomatic in the prior two weeks (healthy controls) or when they had self-reported symptoms of ARI (rhinorrhea, sore throat, cough, difficulty breathing or shortness of breath, wheezing, or cyanosis) in the prior seven days (cases) (Fig. 1 ). Upper respiratory tract illness (URTI) was defined as the presence of any of the following: rhinorrhea, sore throat, or cough. LRTI was defined as the presence of any of the following: difficulty breathing or shortness of breath, wheezing, or cyanosis. At any point in the study, healthy controls were allowed to re-enroll if they developed an ARI, and those initially enrolled with ARI were allowed to re-enroll (1) if they developed a new ARI, or (2) as a healthy control.
Fig. 1.
Enrollment summary for the number of pregnant women (N) in Houston, Texas, 2015–2016. Pregnant women in their second or third trimester of gestation were enrolled if they were asymptomatic (healthy controls) in the prior two weeks or if they had symptoms of ARI in the prior seven days. At any point in the study, pregnant women were allowed to re-enroll. The total number of women initially enrolled with symptoms of ARI was 65. Taking into consideration those 12 healthy controls who re-enrolled when they developed symptoms of ARI, the total number of women with ARI during the study period was 77. The total number of women who re-enrolled was 15.
This study was approved by the Institutional Review Board of Baylor College of Medicine. Written informed consent was obtained. Nasal-pharyngeal secretions and serum samples for both ARI cases and healthy controls were obtained at enrollment. Clinical outcomes were assessed at enrollment and at a two-week phone follow-up. Each subsequent enrollment was treated as a new event, with collection of nasal-pharyngeal secretions and serum samples at re-enrollment, as well as clinical outcomes two weeks later.
2.2. Case definitions, PCR, and serological assays
The rate of PCR-confirmed RSV ARI in pregnant women was calculated by two approaches. The first was based on the participants’ ARI vs. healthy status at first enrollment (65 ARI cases and 90 controls). The second approach was based on the first ARI episode and includes those women who were first enrolled as healthy and subsequently re-enrolled as an ARI case (77 ARI cases and 78 controls).
RSV infection was determined by PCR or serology. ARI was attributed to RSV infection if RSV PCR was positive. Acute RSV infection diagnosed by serology required at least two serum samples more than two weeks apart to observe a four-fold or greater increase in RSV specific neutralizing antibody titer or a Western Blot infection profile. However, because the serum samples did not bracket a particular ARI, the viral cause of the ARI could not be definitively confirmed. When only one serum sample was available that had a Western Blot profile consistent with infection subjects were designated as having had a recent prior RSV infection [21], [22].
PCR: The PCR assays were performed as previously described, in a CLIA certified Respiratory Virus Diagnostic laboratory (CLIA ID# 45D0919666). Nasal-pharyngeal secretions were evaluated for a number of respiratory pathogens, including RSV/A and /B [18], [19], [20].
Western Blot: The RSV-Western Blot assay was utilized to determine serological evidence of a recent prior RSV infection when only one serum sample was available or of an acute serologic infection when two or more serum samples were available for analysis. This was done by evaluating antibody binding patterns to internal [nucleoprotein (N), phosphoprotein (P) and matrix-2 (M2-1)], and surface [attachment (G) and fusion (F)] RSV proteins, as previously described [21], [22]. A recent prior RSV infection was suggested by detecting bands to one or more internal viral proteins in a single serum sample, and an acute serologic RSV infection was identified when there was an increase or new RSV protein band(s) identified in the second serum sample compared to the first serum sample. Previous studies indicate that the banding patterns seen with a recent prior RSV infection suggest that a person has been infected sometime within the last year [21], [22].
Microneutralization Assay: Serum neutralizing antibodies against RSV/A (Tracy) and RSV/B (18537) were quantified using a previously described qualified microneutralization assays [23]. Neutralizing antibody titers were defined as the final dilution at which there was a 50% reduction in viral cytopathic effect (CPE). The lowest limit of detection was 2.5 log2. Any sample resulting in a titer below the lower limit of detection was assigned a value of 2.0 log2. Individuals who developed a four-fold or greater rise in neutralizing antibody titer between the serum samples collected at enrollment and subsequent re-enrollment were reported to have an acute serologic RSV infection.
Palivizumab Competitive Antibody Assay (PCA): A PCA assay was performed as previously described to detect the concentration of palivizumab-like antibodies (PLA) that compete with biotinylated palivizumab for binding to antigenic site II of the fusion protein of RSV [24]. The source of the fusion protein was from sucrose purified RSV/A/Bernett (GA1 genotype) that was coated onto the 96-well plate for 18 h. The lower limit of detection was 1 μg/mL. Those samples with undetectable levels of PLA were assigned a value of 0.5 μg/mL.
2.3. RSV season
Data from the Texas Department of State Health Services were utilized to define the RSV season [26]. For Houston, the 2015–2016 RSV season was defined as the timeframe in which the percentage of positive RSV antigen tests rose above the 10% threshold (onset, week 43) and decline below 10% threshold (end of season, week 12).
2.4. Statistics
A 5–15% incidence of community acquired RSV was used to estimate sample size. A total of 150 or 71 subjects per group were required for 5% or 10% respective incidence rates of RSV infection to show a 5% difference in RSV incidence between RSV-infected pregnant women and healthy pregnant women using a power of 80% and an alpha level of 0.05.
Frequencies of demographic, clinical data, and laboratory findings were determined for RSV-infected and healthy control groups. Frequency distributions between groups were compared using Fisher’s exact test. An independent t-test or Kruskal-Wallis test was conducted to compare mean or median values of continuous demographic and clinical data between groups. The geometric mean ratio (GMR) with a 95% confidence interval was calculated to compare the difference in the geometric mean titers (GMT) of the serologic data between groups. Stata software version 14.2 was used to perform all statistical analyses [25].
3. Results
3.1. Population
At their first enrollment there were 65 pregnant women with ARI and 90 healthy pregnant women controls. Twelve of the ninety healthy pregnant women controls were re-enrolled later during the RSV season with ARI. Similarly two pregnant women with ARI at their initial enrollment were re-enrolled twice more with new ARI.[18].
3.2. PCR positive RSV ARI rates
Among the 65 pregnant women with ARI at their initial enrollment, 39 (60%) had PCR evidence of a respiratory pathogen, of which seven (11%) were PCR-confirmed RSV infections. When expanded to include all women with an ARI during the study period, among 77 enrolled pregnant women with an ARI, 47 (61%) had PCR evidence of a respiratory pathogen, and eight (10%) were PCR-confirmed RSV infections. Thus, the rate of RSV ARI by initial enrollment during the RSV season was 11% (7 of 65) and by ARI episode it was 10% (8 of 77).
3.3. Clinical presentation of PCR positive RSV cases
We compared women with PCR-confirmed RSV infection (n = 8) to pregnant women who were enrolled as healthy controls (n = 90). There were no significant differences between pregnant women with PCR-confirmed RSV and healthy pregnant women controls in regards to demographics or medical history (Table 1 ). Asthma was uncommon in both groups and allergies were reported by three (38%) individuals with PCR-confirmed RSV and 42 (47%) healthy pregnant women. Co-morbidities and complications in current or previous pregnancies were similarly infrequent in both groups.
Table 1.
Demographics and medical history of pregnant women with PCR-confirmed RSV and healthy controls.
| RSV-infected n = 8 |
Healthy n = 90 |
p-value | |
|---|---|---|---|
| Mean age [years] | 32 ± 4 | 30 ± 5 | 0.20 |
| Median gestational age [weeks] | 34 (11) | 28 (9) | 0.14 |
| Median weight [kilograms] | 68 (12) | 68 (23) | 0.78 |
| Median height [centimeters] | 161 (9) | 163 (10) | 0.74 |
| Race | 0.77 | ||
| White | 6 (75) | 64 (71) | |
| Black | 2 (25) | 13 (14) | |
| Asian | 0 | 9 (10) | |
| Other | 0 | 4 (4) | |
| Ethnicity Hispanic or Latino | 2 (25) | 33 (37) | 0.71 |
| Asthma | 1 (13) | 6 (7) | 0.46 |
| Allergies | 3 (38) | 42 (47) | 0.72 |
| Any co-morbidities* | 1 (13) | 20 (22) | >0.99 |
| Thyroid disease | 1 | 9 | |
| Diabetes | 0 | 4 | |
| Other chronic disease | 0 | 9 |
PCR, real-time reverse transcription polymerase chain reaction.
RSV, respiratory syncytial virus.
Data are mean ± standard deviation (SD), Median and interquartile range (IQR), or n (%).
Two healthy controls had two co-morbidities.
All patients with PCR-confirmed RSV reported symptoms of ARI in the week prior to enrollment (Table 2 ). The time post-onset of symptoms at enrollment ranged from 1 day to 9 days, except for one individual who reported 25 days of symptoms. Cough (n = 7/8), congestion (7/8), and sore throat (6/8) were the most common symptoms among those with PCR-confirmed RSV ARI. Three (37.5%) patients reported fever. Four (50%) patients with PCR-confirmed RSV infection reported symptoms of an upper respiratory illness (congestion, sore throat, or cough), and all had symptom onset <6 days prior to enrollment. Four of eight women (50%) with PCR-confirmed RSV had symptoms consistent with a LRTI (shortness of breath or wheezing) and all were ≥6 days post-onset of symptoms. These four individuals also reported fever, chest pain, nausea or diarrhea, loss of appetite, and decreased activity with a greater frequency (Table 2). All women with RSV-LRTI also reported a previous ARI during their pregnancy, one of which developed into pneumonia.
Table 2.
Case summaries of pregnant women with PCR-confirmed RSV.
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 |
|---|---|---|---|---|---|---|---|---|
| Age [years] | 31 | 28 | 26 | 33 | 35 | 28 | 35 | 37 |
| Gestation [weeks] | 15 | 24 | 39 | 37 | 34 | 34 | 35 | 26 |
| Comorbidity/Risk Factors | NR | NR | NR | Hypothyroidism | NR | NR | Asthma, twins | NR |
| Virus | RSV-A | RSV-A | RSV-B | RSV-A | RSV-A, HRV | RSV-A | RSV-A | RSV-A |
| Symptoms | Sore throat Decreased activity Decreased appetite |
Congestion Cough Sore throat |
Congestion Cough Sneezing |
Congestion Cough Sore throat |
Congestion Cough Decreased activity Fever Short of breath |
Congestion Cough Sore throat Short of breath |
Congestion Cough Sore throat Decreased activity Decreased appetite Fever Short of breath Chest pain Wheezing Diarrhea Vomiting Contractions |
Congestion Cough Sore throat Decreased activity Decreased appetite Fever Short of breath Chest pain Wheezing Diarrhea |
| Medications | NR | NR | NR | Antihistamine | NR | Cough drops | Antibiotics, cough medicine, pain reliever | Inhaled glucocorticoid, antibiotics, pain reliever |
| Gravida [n], Para [n] | 3, 2 | 2, 1 | 3, 2 | 1, 0 | 2, 1 | 1, 0 | 5, 1 | 3, 2 |
| Healthcare Facility Utilized | Clinic | Clinic | Clinic | Clinic | Clinic | Clinic | Hospital | Clinic |
| Duration of Illness [days] | 9 | 7 | 11 | 18 | Still reporting at two weeks follow-up | Still reporting at two weeks follow-up | 14 | 30 |
PCR, real-time reverse transcription polymerase chain reaction.
RSV, respiratory syncytial virus.
HRV, human rhinovirus.
NR, none reported.
Most women with PCR-confirmed RSV ARI were seen at the obstetrics and gynecology clinic, and one patient was hospitalized. This patient presented during the peak of the RSV season with sudden onset of fever and uterine contractions. She was a 35-year-old, gravida 5, para 1, 35 weeks of gestation with dichorionic diamniotic twins. The physical exam was significant for left costovertebral angle and flank pain; she reported general malaise. The patient was started on antibiotics and supportive care. During her stay she developed symptoms of LRTI and was treated with azithromycin. Within 48 h, symptoms improved and the patient was discharged. She was enrolled into the study five days after hospital discharge and was still reporting congestion, cough, and sore throat. These symptoms resolved 10 days after discharge.
The duration of illness for women with PCR-confirmed RSV ranged from 7 to 30 days. In general, those who were enrolled later in their illness (≥6 days) experienced a longer illness. Two women (25%) continued to report symptoms when contacted at their two-week follow-up (Table 2). Four (50%) reported the use of over-the-counter medications, including antihistamines, cough medicine, and pain relievers. Two (25%) were prescribed medications, including antibiotics and inhaled glucocorticoid.
3.4. RSV cases identified by serology
Of the three pregnant women with ARI at their initial enrollment who subsequently re-enrolled in the study, one exhibited an acute serologic RSV infection profile. In addition, of the twelve women initially enrolled as healthy controls who later re-enrolled in the study with ARI symptoms, two exhibited an acute serologic RSV infection profile (Table 3 ). In total, 3 (20%) of 15 pregnant women had serologic evidence of RSV infection. None of these individuals had PCR-confirmed RSV. One woman (Case 9) was initially enrolled as healthy control, but developed symptoms of an ARI by her two-week follow-up, and was secondarily enrolled as an ARI case. Similarly, Case 10 was also enrolled as healthy control and reported symptoms of ARI at two weeks. However, she was not re-enrolled into the study until 30 days after her first enrollment when she reported an additional instance of ARI. Case 11 was initially enrolled with ARI and 7 weeks later enrolled a second time as a healthy control.
Table 3.
Case summaries of pregnant women with a serologic evidence of an acute RSV infection.
| Variables | Case 9 | Case 10 | Case 11 |
|---|---|---|---|
| Age [years] | 30 | 28 | 23 |
| Gestation [weeks] | 27 | 16 | 33 |
| Comorbidity/Risk Factors | Gestational diabetes | NR | NR |
| First Enrollment | |||
| Serum Collected [CDC week] | 44 | 48 | 45 |
| Time Post-Onset [days] | 4 | 5 | 2 |
| Virus | HRV | Negative | Negative |
| Neutralizing Antibodies RSV/A, RSV/B [titer] | 64, 181 | 512, 45 | 362, 90 |
| PLA concentration [μg/mL] | 0.9 | 18.8 | 3.3 |
| Symptoms | Congestion Cough Sore throat Sneezing |
Congestion Sore throat Sneezing |
Congestion Cough Sore throat Decreased activity Short of breath Wheezing |
| Duration [days] | Still reporting at two weeks follow-up | Still reporting at two weeks follow-up | Still reporting at two weeks follow-up |
| Second Enrollment | |||
| Serum Collected [CDC week] | 46 | 1 | 52 |
| Time Post-Onset [days] | 4 | 4 | NA |
| Virus | HCoV | Negative | Negative |
| Neutralizing Antibodies RSV/A, RSV/B [titer] | 128, 128 | 362, 45 | 256, 64 |
| PLA concentration [μg/mL] | 8.5 | 5.4 | 4.4 |
| Symptoms | Congestion Cough Sneezing Decreased activity Wheezing |
Congestion Cough Sore throat Decreased appetite Chest pain Wheezing |
NA |
| Duration [days] | 5 | Still reporting at two weeks follow-up | NA |
RSV, respiratory syncytial virus.
HRV, human rhinovirus.
PLA, palivizumab-like antibodies.
HCoV, human coronavirus.
NR, none reported.
NA, as this patient was enrolled as healthy, variables referring to illness are not applicable.
In combining the PCR and serology data, the RSV attack rate based on health status at initial enrollment during the RSV season was 12% (8 of 65) and by ARI episode it was 13% (10 of 77).
3.5. Recent prior RSV infection by Western Blot
The majority of patients did not re-enroll in the study and, as a result, only had one serum sample available for Western Blot analysis. Of the eight patients with PCR-confirmed RSV, six did not have Western Blot evidence of a recent prior RSV infection (Table 4 ). These six individuals were enrolled early in their infection (1–8 days of illness onset) and therefore we did not expect to observe an antibody response to their RSV infection. Two patients with PCR-confirmed RSV had serologic evidence of a recent prior RSV infection profile; these two individuals were enrolled on days 9 and 25 of their illness onset. Of the 88 healthy controls enrolled during the RSV season, 28 (31%) had a Western Blot profile consistent with recent prior RSV infection.
Table 4.
Serological results of PCR-confirmed RSV cases by onset of illness compared to healthy controls.
| Variables | <6 Days RSV Illness n = 4 |
≥6 Days RSV Illness n = 4 |
RSV-infected n = 8 |
Healthy n = 90 |
RSV vs. Healthy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study case | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |||
| Time Post-Onset [days] | 1 | 2 | 2 | 5 | 6 | 8 | 9 | 25 | |||
| Serum Collected [CDC week] | 51 | 46 | 45 | 48 | 44 | 14 | 46 | 1 | |||
| Recent Prior RSV Infection Profile by WB | No | No | No | No | No | No | Yes | Yes | 2 (25)* | 28 (31)* | P > 0.99* |
| PLA concentration [μg/mL] | 10.4 | 5.4 | 0.9 | 3.9 | 4.7 | 5.0 | 25.3 | 10.5 | 5.8 (2.6–13.0)† |
6.4 (5.5–7.4)† |
0.9 (0.4–2.0)‡ |
| RSV/A Neutralizing Antibodies [titer] | 128 | 512 | 64 | 181 | 256 | 181 | 724 | 512 | 245.1 (123.8–485.2)† | 173.5 (146.4–205.6)† | 1.4 (0.7–2.8)‡ |
| RSV/B Neutralizing Antibodies [titer] | 256 | 128 | 64 | 128 | 362 | 128 | 1024 | 724 | 234.8 (105.4–523.0)† | 171.7 (134.8–218.5)† | 1.4 (0.6–3.1)‡ |
PCR, real-time reverse transcription polymerase chain reaction.
RSV, respiratory syncytial virus.
WB, Western Blot.
PLA, palivizumab-like antibodies.
Data are reported as n (%) and p-value.
Data are reported as geometric mean (95% CI).
Data are reported as geometric mean ratio of RSV-infected/Healthy (95% CI).
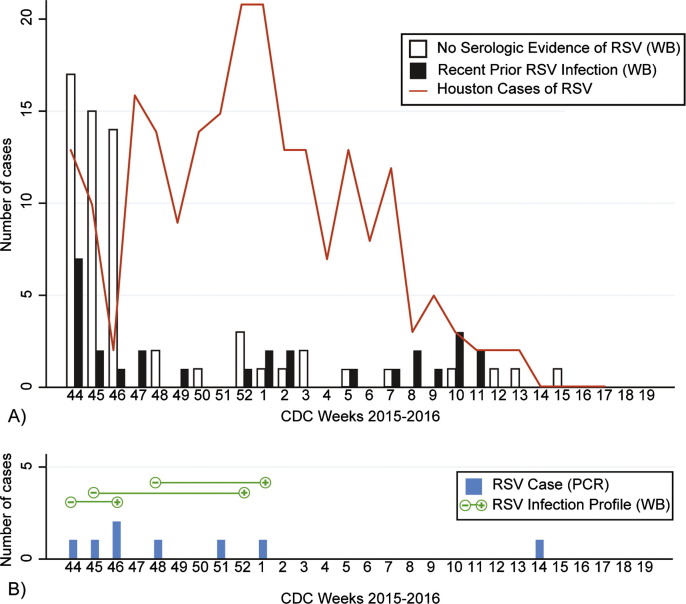
It would be expected that if RSV infection occurred during the RSV season, the greatest proportion of women with a recent prior RSV infection would occur later into the season when (1) greater exposure to RSV has occurred, and (2) time to develop an immune response following the RSV infection has elapsed. In Houston, the RSV season began on CDC week 43 and peaked weeks 3–4, ending week 12 (Fig. 2 ). Overall, the rate of patient enrollment was greatest in the first third of the RSV season (weeks 43–50). The proportion of healthy controls with serologic evidence of a recent prior RSV infection significantly increased from 21% (13/62) in the first third of the RSV season, to 43% (6/14) in the second third, and 64% (9/14) in the last third (p < 0.004). In addition, two healthy controls were enrolled shortly after the end of the RSV season (weeks 13 and 15) and are included in Fig. 2.
Fig. 2.
Respiratory syncytial virus (RSV) laboratory results for pregnant women in Houston, Texas, 2015–2016. (A) Presence or absence of a recent prior RSV infection Western Blot profile for healthy pregnant women (n = 90) by CDC Week. The red line indicates the total positive RSV antigen tests for the Houston area as reported by the Texas Department of State Health Services. (B) Pregnant women with PCR-confirmed RSV (n = 8) and RSV infection profile (n = 3) by CDC Week. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Serum neutralizing antibody and Palivizumab-Like Antibody (PLA) results
Healthy controls had serum neutralizing antibody GMT of 173.5 for RSV/A and171.7 for RSV/B, while the GMTs for women with PCR-confirmed RSV were 245.1 and 234.8, respectively. The two women with PCR-confirmed RSV who were enrolled on days 9 and 25 (Table 4) of their illness had RSV/A neutralizing antibody titer (reciprocal value) of 724 and 512 and RSV/B neutralizing antibody levels of 1024 and 724. In comparing healthy pregnant women controls with no serologic evidence of RSV infection (n = 62) and healthy pregnant women controls with a recent prior RSV infection profile (n = 28), the GMT of neutralizing antibodies for RSV/A (167.4, 95% CI: 133.7–209.5 versus 187.9, 95% CI: 147.3–239.7) and RSV/B (167.4, 95% CI: 123.6–226.7, versus 181.5, 95% CI: 119.8–274.9) were similar.
The geometric mean of PLA concentration of healthy controls (6.4 µg/mL) was similar to that of women with PCR-confirmed RSV (5.8 µg/mL). However, those two individuals with PCR-confirmed RSV infection who were enrolled later in their illness (days 9 and 25) had comparatively greater levels of PLA, 25.3 μg/mL and 10.5 μg/mL respectively.
4. Discussion
PCR-confirmed RSV contributed to 10 or 11% of ARI cases based on initial enrollment status or first ARI episode, respectively, among pregnant women in our study. Because we enrolled women seen at an outpatient clinic regardless of history of fever, we were able to capture the full range of clinical manifestations of RSV infection in pregnancy without a selection bias for severe or concurrent disease. We found that 50% (4/8) of the pregnant women with PCR-confirmed RSV had symptoms of LRTI; this included one woman who was hospitalized, possibly related to her RSV illness.
Previous studies have documented the combined diagnostic strength of PCR and serology [26], [27]. Utilizing both techniques we identified RSV infection in acute samples by PCR and convalescent samples by serology. RSV was identified by PCR in 11% (7/65) of pregnant women with symptoms of ARI at their initial enrollment. Taking into account one additional woman who exhibited an acute serologic RSV infection, the RSV attack rate was at least 12% (8/65) of ARI cases. An RSV attack rate as high as 13% (10/77) is estimated if calculated using the first ARI episode. This is substantial given that the RSV season in Houston was relatively mild compared to previous years [28], and greater than reported by maternal studies of respiratory illness in Nepal and Mongolia, where RSV accounted for 2% and 2.4% of ARI cases, respectively [14], [15]. However, because fever was an inclusion criteria for those studies, and fever occurs in less than one-third of adult RSV infections, these numbers may be an underestimate [29]. Our attack rate of 12–13% is similar to that of other studies with broad ARI inclusion criteria in adults. In a four year study of ARI in high risk adults, RSV was detected in 46 of 519 (10%) cases [9]. Similarly, a 20-year study of otherwise healthy adults found that 7% of individuals with respiratory illness were infected with RSV [30].
Nearly one-third (32%, 28/88) of healthy pregnant women enrolled during the RSV season had evidence of a Western Blot profile consistent with a recent prior RSV infection and, based on serology, 2 of 12 (16.7%) healthy pregnant women went on to develop an RSV infection during the RSV season. Recent studies have reported findings wherein at least 20% of women of childbearing age had serologic evidence of an acute RSV infection [21], [31]. In our study, the percentage of healthy pregnant women with a Western Blot profile of a recent prior RSV infection increased significantly as the RSV season progressed. These women had enrollment dates that followed the peak of RSV illness in Houston. This suggests that these healthy controls may have been infected with RSV during the course of this study. A caveat in our method of defining the RSV season is the use of the RSV antigen test as a marker for infection. A recent study found that utilizing PCR as an alternative diagnostic tool allows for a more expanded RSV season and, as a result, an increase in the RSV cases reported [32].
RSV infection during pregnancy resulted in a range of clinical manifestation of ARI illnesses. Cough, congestion, and sore throat were the most common symptoms among those with PCR-confirmed RSV. In previous studies of ILI, pregnant women with RSV generally reported a mild, febrile illness restricted to the upper airways [14], [15]. In our study women who reported fever also had symptoms of LRTI. Although none of the women were diagnosed with pneumonia, the morbidity attributable to RSV was significant. One woman did report symptoms of LRTI during hospitalization for fever and uterine contractions and was found to have PCR-confirmed RSV several days after hospital discharge. She, like those in the case-series by Wheeler et al, reported co-morbidities that may have put her at risk for a more serious illness [13].
Due to differences in study design and sample collection, it is difficult to make comparisons between our study and others that have documented maternal RSV infection. Three of the previous studies were prospective, wherein a participant was actively followed during the RSV season by weekly visits or telephone calls, sampled when she became ill, and followed for clinical outcomes through the duration of her pregnancy [14], [15], [16]. However, these study participants were only sampled when they reported ILI or sought medical attention for their respiratory illness. The retrospective case-series describes three patients identified through tertiary care records [13]. These studies may select for a more severe illness or illness restricted to ILI. Conversely, in our study we captured women in different stages of their illness.
Of the eight women with PCR-confirmed RSV, only two had serological evidence of a recent prior RSV infection by Western Blot. Both were enrolled well into their illness, at 9 and 25 days, giving them time to develop an RSV specific immunological response. This is also evident by their comparatively greater levels of neutralizing antibodies and palivizumab-like antibodies [9], [33]. Three women who were re-enrolled and had paired sera, exhibited an RSV infection profile despite negative PCR. It is likely that these women were exposed to RSV sometime between their first and second enrollment visit. Although we cannot definitively establish causality, it is possible that RSV infection was responsible for some or all of the symptoms these three women reported. Twenty-eight healthy controls had serologic evidence of a recent prior RSV infection by Western Blot testing of a single serum sample, suggesting they had been infected with RSV at sometime within the year. The proportion of healthy controls with a Western blot profile of a recent prior RSV infection significantly increased in the latter part of the RSV season. This suggests that many of these healthy women had been infected with RSV earlier in the current RSV season and, that by the time of study enrollment, sufficient time had elapsed to develop an RSV-specific antibody response.
Our study is limited in its size and duration. Because patients were enrolled at a single site over one RSV season, we were only able to capture eight women with PCR-confirmed RSV. However, this proportion is greater than that reported by other studies to date and, given our broad inclusion criteria, we were able to describe a range of RSV clinical manifestations. The case definition is limited in that it is dependent on self-reported symptoms, and LRTI was not confirmed by radiologic study. In addition, because serum samples were collected at the time of enrollment, only one serum sample was available for most patients, unless participants chose to re-enroll in the study at a later time. Given this, RSV case identification by serology was dependent on the number of serum samples provided: patients with sequential serum samples could be identified as having an acute serologic RSV infection, while patients with a single serum sample could be identified as having had a recent prior RSV infection.
In summary, RSV is a common cause of ARI during pregnancy with appreciable morbidity. Although the primary goal of maternal immunization against RSV is to protect young infants who are at increased risk for severe disease, a maternal RSV vaccine may also directly benefit pregnant women. As maternal RSV vaccine candidates move forward in evaluation, additional priority should be placed on evaluating the impact of RSV disease prevention in pregnant women.
Acknowledgments
Acknowledgements
We would like to thank the office of Woman’s OB/GYN Specialists and all of the women who participated in the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.04.098.
Contributor Information
Anne M. Hause, Email: anne.hause@emory.edu.
Pedro A. Piedra, Email: ppiedra@bcm.edu.
Flor M. Munoz, Email: florm@bcm.edu.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Beigi R.H. Prevention and management of influenza in pregnancy. Obstet Gynecol Clin North Am. 2014;41:535–546. doi: 10.1016/j.ogc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Steinhoff M.C., Omer S.B., Roy E., Arifeen S.E., Raqib R., Dodd C. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. Can Med Assoc J. 2012;184:645–653. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207:S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Grohskopf L.A., Sokolow L.Z., Broder K.R., Walter E.B., Bresee J.S., Fry A.M. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2017–18 Influenza Season. MMWR Recomm Reports. 2017;66:1–20. doi: 10.15585/mmwr.rr6602a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013;62:131–5. [PMC free article] [PubMed]
- 6.Munoz F.M., Englund J.A., Cheesman C.C., Maccato M.L., Pinell P.M., Nahm M.H. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20:826–837. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 7.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi T., McAllister D.A., O’Brien K.L., Simoes E.A.F., Madhi S.A., Gessner B.D. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet (London, England) 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 10.Chu H.Y., Steinhoff M.C., Magaret A., Zaman K., Roy E., Langdon G. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant Pairs in Bangladesh. J Infect Dis. 2014;210:1582–1589. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyiro J.U., Sande C., Mutunga M., Kiyuka P.K., Munywoki P.K., Scott J.A.G. Quantifying maternally derived respiratory syncytial virus specific neutralising antibodies in a birth cohort from coastal Kenya. Vaccine. 2015;33:1797–1801. doi: 10.1016/j.vaccine.2015.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suara R.O., Piedra P.A., Glezen W.P., Adegbola R.A., Weber M., Mulholland E.K. Prevalence of neutralizing antibody to respiratory syncytial virus in sera from mothers and newborns residing in the Gambia and in The United States. Clin Diagn Lab Immunol. 1996;3:477–479. doi: 10.1128/cdli.3.4.477-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler S.M., Dotters-Katz S., Heine R.P., Grotegut C.A., Swamy G.K. Maternal effects of respiratory syncytial virus infection during pregnancy. Emerg Infect Dis. 2015;21:1951–1955. doi: 10.3201/eid2111.150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu H.Y., Katz J., Tielsch J., Khatry S.K., Shrestha L., LeClerq S.C. Clinical presentation and birth outcomes associated with respiratory syncytial virus infection in pregnancy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaw L., Kamigaki T., Burmaa A., Urtnasan C., Od I., Nyamaa G. Burden of influenza and respiratory syncytial virus infection in pregnant women and infants under 6 months in Mongolia: a prospective cohort study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madhi S.A., Cutland C.L., Downs S., Jones S., van Niekerk N., Simoes E.A.F. Burden of respiratory syncytial virus infection in South African HIV-infected and HIV-uninfected pregnant and post-partum women: a longitudinal cohort study. Clin Infect Dis. 2017 doi: 10.1093/cid/cix1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan A.K., Klein N.P., Langley G., Drews S.J., Buchan S., Ball S. Respiratory syncytial virus hospitalization during pregnancy in four high-income Countries, 2010–2016. Clin Infect Dis. 2018 doi: 10.1093/cid/ciy439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hause A.M., Avadhanula V., Maccato M.L., Pinell P.M., Bond N., Santarcangelo P. A cross-sectional surveillance study of the frequency and etiology of acute respiratory illness among pregnant women. J Infect Dis. 2018;218:528–535. doi: 10.1093/infdis/jiy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckham J.D., Cadena A., Lin J., Piedra P.A., Glezen W.P., Greenberg S.B. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50:322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansbach J.M., Piedra P.A., Stevenson M.D., Sullivan A.F., Forgey T.F., Clark S. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics. 2012;130:e492–e500. doi: 10.1542/peds.2012-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.August A., Glenn G.M., Kpamegan E., Hickman S.P., Jani D., Lu H. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35:3749–3759. doi: 10.1016/j.vaccine.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 22.Piedra P.A., Cron S.G., Jewell A., Hamblett N., McBride R., Palacio M.A. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21:2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 23.Piedra P.A., Jewell A.M., Cron S.G., Atmar R.L., Paul Glezen W. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine. 2003;21:3479–3482. doi: 10.1016/s0264-410x(03)00355-4. [DOI] [PubMed] [Google Scholar]
- 24.Ye X., Iwuchukwu O.P., Avadhanula V., Aideyan L.O., McBride T.J., Ferlic-Stark L.L. Comparison of palivizumab-like antibody binding to different conformations of the RSV F protein in RSV-infected adult hematopoietic cell transplant recipients. J Infect Dis. 2018;217:1247–1256. doi: 10.1093/infdis/jiy026. [DOI] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release 14 2015.
- 26.Luchsinger V., Piedra P.A., Ruiz M., Zunino E., Martinez M.A., Machado C. Role of neutralizing antibodies in adults with community-acquired pneumonia by respiratory syncytial virus. Clin Infect Dis. 2012;54:905–912. doi: 10.1093/cid/cir955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falsey A.R., Formica M.A., Walsh E.E. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–820. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Texas Department of State Health Services. Respiratory Syncytial Virus Home 2017 <http://www.dshs.texas.gov/idcu/disease/rsv/> (accessed February 28, 2018).
- 29.Hall C.B., Geiman J.M., Biggar R., Kotok D.I., Hogan P.M., Douglas R.G. Respiratory syncytial virus infections within families. N Engl J Med. 1976;294:414–419. doi: 10.1056/NEJM197602192940803. [DOI] [PubMed] [Google Scholar]
- 30.Hall C.B., Long C.E., Schnabel K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33:792–796. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 31.Glenn G.M., Fries L.F., Thomas D.N., Smith G., Kpamegan E., Lu H. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis. 2016;213:411–422. doi: 10.1093/infdis/jiv406. [DOI] [PubMed] [Google Scholar]
- 32.Midgley C.M., Haynes A.K., Baumgardner J.L., Chommanard C., Demas S.W., Prill M.M. Determining the seasonality of respiratory syncytial virus in the United States: the impact of increased molecular testing. J Infect Dis. 2017;216:345–355. doi: 10.1093/infdis/jix275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye X., Iwuchukwu O.P., Avadhanula V., Aideyan L.O., McBride T.J., Ferlic-Stark L.L. Comparison of palivizumab like antibody binding to different conformations of the RSV F protein in RSV infected adult hematopoietic cell transplant recipients. J Infect Dis. 2018 doi: 10.1093/infdis/jiy026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.