Table 1.
Rate and equilibrium constants of ligand-substitution reactions in acetone [72].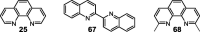
| Ligand-substitution reaction | k298/M−1 s−1 | log K |
|---|---|---|
| [Cu(68)]+ + 68 → [Cu(68)2]+ | 4 × 107 (in MeCN) | 7.0 |
| [Cu(67)2]+ + 68 → [Cu(67)(68)]+ + 67 | 1.8 × 103 | 3.7 |
| [Cu(67)2]+ + 25 → [Cu(25)(67)]+ + 67 | 3.8 × 103 | 2.2 |
| [Cu(67)2]+ + MeCN → [Cu(67)(MeCN)]+ + 67 | 1 × 102 | ≈-4.4 |
| [Cu(67)2]+ + I− → [Cu(67)(I)] + 67 | 6 × 105 | ≈1.2 |
| [Cu(67)(I)] + 68 → [Cu(67)(68)]+ + I− | 2 × 106 | ≈2.5 |
| [Cu(67)(MeCN)]+ + 25 → [Cu(25)(67)]+ + MeCN | ≈1 × 107 |
